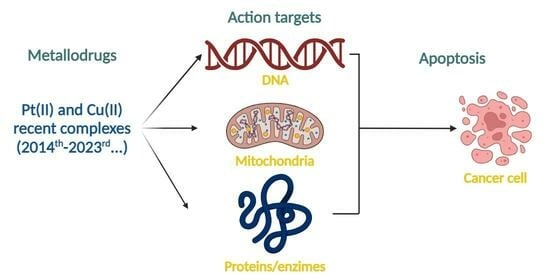
Recently Reported Biological Activities and Action Targets of Pt(II)- and Cu(II)-Based Complexes
Abstract
:1. Introduction
2. General Results of the Meta-Analysis
3. Mechanisms of Action of Platinum(II) and Copper(II) Complexes
3.1. Platinum(II)
3.2. Copper(II)
3.3. DNA Binding Modes of Metal-Based Complexes
4. Recent Studies on Platinum(II) and Copper(II) Complexes and Their Target Sites
4.1. Platinum(II)
4.1.1. Target Site: DNA
4.1.2. Target Site: Mitochondria
4.1.3. Target Site: Proteins and Enzymes
4.2. Copper(II)
4.2.1. Target Site: DNA
4.2.2. Target Site: Mitochondria
4.2.3. Target Site: Proteins and Enzymes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ott, I.; Gust, R. Non Platinum Metal Complexes as Anti-Cancer Drugs. Arch. Pharm. Chem. Life Sci. 2007, 340, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Tolbatov, I.; Marrone, A. Computational Strategies to Model the Interaction and the Reactivity of Biologically-Relevant Transition Metal Complexes. Inorganica Chim. Acta 2022, 530, 120686. [Google Scholar] [CrossRef]
- Wenzel, M.; Casini, A. Mass Spectrometry as a Powerful Tool to Study Therapeutic Metallodrugs Speciation Mechanisms: Current Frontiers and Perspectives. Coord. Chem. Rev. 2017, 352, 432–460. [Google Scholar] [CrossRef]
- Soldevila-Barreda, J.J.; Sadler, P.J. Approaches to the Design of Catalytic Metallodrugs. Curr. Opin. Chem. Biol. 2015, 25, 172–183. [Google Scholar] [CrossRef]
- Ferraro, M.G.; Piccolo, M.; Misso, G.; Santamaria, R.; Irace, C. Bioactivity and Development of Small Non-Platinum Metal-Based Chemotherapeutics. Pharmaceutics 2022, 14, 954. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Model List of Essential Medicines—23rd List. 2023. Available online: Https://Www.Who.Int/Publications/i/Item/WHO-MHP-HPS-EML-2023.02. (accessed on 29 October 2023).
- Comisión Federal Para La Protección Contra Riesgos Sanitarios (COFEPRIS). Listados de Registros Sanitarios de Medicamentos. Available online: https://Tramiteselectronicos02.Cofepris.Gob.Mx/BuscadorPublicoRegistrosSanitarios/BusquedaRegistroSanitario.Aspx (accessed on 29 October 2023).
- Linares, J.; Sallent-Aragay, A.; Badia-Ramentol, J.; Recort-Bascuas, A.; Méndez, A.; Manero-Rupérez, N.; Re, D.L.; Rivas, E.I.; Guiu, M.; Zwick, M.; et al. Long-Term Platinum-Based Drug Accumulation in Cancer-Associated Fibroblasts Promotes Colorectal Cancer Progression and Resistance to Therapy. Nat. Commun. 2023, 14, 746. [Google Scholar] [CrossRef] [PubMed]
- Dilruba, S.; Kalayda, G.V. Platinum-Based Drugs: Past, Present and Future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Monneret, C. Platinum Anticancer Drugs. From Serendipity to Rational Design. Ann. Pharm. Françaises 2011, 69, 286–295. [Google Scholar] [CrossRef]
- Da Silva, D.A.; De Luca, A.; Squitti, R.; Rongioletti, M.; Rossi, L.; Machado, C.M.L.; Cerchiaro, G. Copper in Tumors and the Use of Copper-Based Compounds in Cancer Treatment. J. Inorg. Biochem. 2022, 226, 111634. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Ji, Z.; Xu, X.; Zhang, H.; Wang, Y. Polysaccharide-platinum Complexes for Cancer Theranostics. Carbohydr. Polym. 2023, 315, 120997. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Chai, K.; Jiang, Y.; Han, T.; Niu, J.; Yao, L.; Zhang, H.; Zeng, M.; Zhang, L.; Duan, X.; Wang, J. Synthesis, DNA Binding, Topoisomerase I Inhibition and Antiproliferation Activities of Three New Binuclear Terpyridine Platinum(II) Complexes. Polyhedron 2019, 157, 124–130. [Google Scholar] [CrossRef]
- Liu, J.-H.; Wei, Z.-Z.; Yang, L.; Qin, Q.-P.; Liu, X.-X.; Tan, M.-X. Synthesis, Structures, and Anticancer Potentials of Four Platinum (II) Complexes with Benzopyran Derivatives Targeting Mitochondria. Inorg. Chem. Commun. 2020, 122, 108267. [Google Scholar] [CrossRef]
- Wei, Q.-M.; Wei, Z.-Z.; Zeng, J.-J.; Yang, L.; Qin, Q.-P.; Tan, M.-X.; Liang, H. Synthesis, Structures and Anticancer Potentials of Five Platinum(II) Complexes with Benzothiazole-Benzopyran Targeting Mitochondria. Polyhedron 2021, 196, 115004. [Google Scholar] [CrossRef]
- Moreira, R.O.; Morcelli, S.R.; Kanashiro, M.M.; Resende, J.A.L.C.; Maciel, L.L.F.; Almeida, J.C.D.A.; Gahan, L.R.; Horn, A.; Fernandes, C. Modulating the Antitumoral Activity by the Design of New Platinum(II) Compounds: Synthesis, Characterization, DFT, Ultrastructure and Mechanistic Studies. J. Inorg. Biochem. 2019, 194, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.L.; Stagno, C.; Efferth, T.; Omer, E.A.; D’Angelo, V.; Germanò, M.P.; Cacciola, A.; De Gaetano, F.; Iraci, N.; Micale, N. Antileukemia Activity and Mechanism of Platinum(II)-Based Metal Complexes. Molecules 2022, 27, 9000. [Google Scholar] [CrossRef]
- Bivián-Castro, E.Y.; Roitzsch, M.; Gupta, D.; Lippert, B. Synthesis and X-Ray Crystal Structure Analysis of 1:1 and 1:2 Complexes of Cisplatin with the Model Nucleobase 9-Methyladenine in Its Protonated Form and a Unique HNO3 Adduct of Cis-[(NH3)2Pt(9-MeAH-N7)2]4+. Inorganica Chim. Acta 2005, 358, 2395–2402. [Google Scholar] [CrossRef]
- Maciel, L.L.F.; Silva, M.B.; Moreira, R.O.; Cardoso, A.P.; Fernandes, C.; Horn, A.; De Aquino Almeida, J.C.; Kanashiro, M.M. In Vitro and In Vivo Relevant Antineoplastic Activity of Platinum(II) Complexes toward Triple-Negative MDA-MB-231 Breast Cancer Cell Line. Pharmaceutics 2022, 14, 2013. [Google Scholar] [CrossRef]
- Abolhassan, M.R.; Divsalar, A.; Badalkhani-khamseh, F.; Kheiripour, N.; Eslami-Moghadam, M.; Mirzaei, H. Protein Binding and Anticancer Activity of Two Newly Synthesized Schiff Base Platinum (II) Complexes: A Theoretical and Experimental Study. J. Mol. Struct. 2023, 1289, 135917. [Google Scholar] [CrossRef]
- Imran, M.; Zia-ur-Rehman; Kondratyuk, T.; Kondratyuk, F. New Ternary Platinum(II) Dithiocarbamates: Synthesis, Characterization, Anticancer, DNA Binding and DNA Denaturing Studies. Inorg. Chem. Commun. 2019, 103, 12–20. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Yuan, C.; Su, F.; Wu, Y.-B.; Lu, L.; Zhu, M.; Xing, S.; Fu, X. Syntheses, Crystal Structures, and Biological Evaluations of New Dinuclear Platinum(ii) Complexes with 1,2,4-Triazole Derivatives as Bridging Ligands. Dalton Trans. 2021, 50, 4527–4538. [Google Scholar] [CrossRef]
- Reina, M.; Hernández-Ayala, L.F.; Bravo-Gómez, M.E.; Gómez, V.; Ruiz-Azuara, L. Second Generation of Casiopeinas®: A Joint Experimental and Theoretical Study. Inorganica Chim. Acta 2021, 517, 120201. [Google Scholar] [CrossRef]
- Das, K.; Dolai, S.; Vojtíšek, P.; Manna, S.C. Protein Binding, DFT/TDDFT Calculation and Catecholase Activity of Five Coordinated Distorted Square Pyramidal/Trigonal Bipyramidal Cu(II) Complexes. Polyhedron 2018, 149, 7–16. [Google Scholar] [CrossRef]
- Sahu, R.; Padhi, S.K.; Jena, H.S.; Manivannan, V. Conversion of 2-(Aminomethyl) Substituted Pyridine and Quinoline to Their Dicarbonyldiimides Using Copper(II) Acetate. Inorganica Chim. Acta 2010, 363, 1448–1454. [Google Scholar] [CrossRef]
- Choi, K.-Y.; Kim, B.-R.; Ko, J. Synthesis, Properties, and Crystal Structures of Copper(II) Di-(2-Picolyl)Amine Complexes Containing Inorganic Salts. J. Chem. Crystallogr. 2007, 37, 847–852. [Google Scholar] [CrossRef]
- Puttock, E.V.; Sturala, J.; Kistemaker, J.C.M.; Williams, J.A.G. Platinum(II) Complexes of Tridentate-Coordinating Ligands Based on Imides, Amides, and Hydrazides: Synthesis and Luminescence Properties. Eur. J. Inorg. Chem. 2021, 2021, 335–347. [Google Scholar] [CrossRef]
- Bai, L.; Gao, C.; Liu, Q.; Yu, C.; Zhang, Z.; Cai, L.; Yang, B.; Qian, Y.; Yang, J.; Liao, X. Research Progress in Modern Structure of Platinum Complexes. Eur. J. Med. Chem. 2017, 140, 349–382. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-DePaz, Y.; Resendiz-Acevedo, K.; Dávila-Manzanilla, S.G.; García-Ramos, J.C.; Ortiz-Frade, L.; Serment-Guerrero, J.; Ruiz-Azuara, L. DNA, a Target of Mixed Chelate Copper(II) Compounds (Casiopeinas®) Studied by Electrophoresis, UV–Vis and Circular Dichroism Techniques. J. Inorg. Biochem. 2022, 231, 111772. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, S.A.; Badr, H.E.; Di Biase, A.; El-Hendawy, A.M. Synthesis, Characterization of Ruthenium(II), Nickel(II), Palladium(II), and Platinum(II) Triphenylphosphine-Based Complexes Bearing an ONS-Donor Chelating Agent: Interaction with Biomolecules, Antioxidant, in Vitro Cytotoxic, Apoptotic Activity and Cell Cycle Analysis. J. Inorg. Biochem. 2021, 223, 111549. [Google Scholar] [CrossRef]
- Mbugua, S.N.; Sibuyi, N.R.S.; Njenga, L.W.; Odhiambo, R.A.; Wandiga, S.O.; Meyer, M.; Lalancette, R.A.; Onani, M.O. New Palladium(II) and Platinum(II) Complexes Based on Pyrrole Schiff Bases: Synthesis, Characterization, X-Ray Structure, and Anticancer Activity. ACS Omega 2020, 5, 14942–14954. [Google Scholar] [CrossRef]
- Zalba, S.; Garrido, M.J. Liposomes, a Promising Strategy for Clinical Application of Platinum Derivatives. Expert Opin. Drug Deliv. 2013, 10, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Apps, M.G.; Choi, E.H.Y.; Wheate, N.J. The State-of-Play and Future of Platinum Drugs. Endocr. Relat. Cancer 2015, 22, R219–R233. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Guo, Z. Functionalization of Platinum Complexes for Biomedical Applications. Acc. Chem. Res. 2015, 48, 2622–2631. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting Copper in Cancer Therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef] [PubMed]
- Krstic, N.; Nikolic, R.; Stankovic, M.; Nikolic, N.; Dordevic, D. Coordination Compounds of M(II) Biometal Ions with Acid- Type Anti-Inflammatory Drugs as Ligands—A Review. Trop. J. Pharm. Res. 2015, 14, 337. [Google Scholar] [CrossRef]
- Balsa, L.M. Copper Complexes as Antitumor Agents: In Vitro and In Vivo Evidence. Curr. Med. Chem. 2023, 30, 510–557. [Google Scholar] [CrossRef]
- Bivián-Castro, E.Y.; López, M.G.; Pedraza-Reyes, M.; Bernès, S.; Mendoza-Díaz, G. Synthesis, Characterization, and Biological Activity Studies of Copper(II) Mixed Compound with Histamine and Nalidixic Acid. Bioinorg. Chem. Appl. 2009, 2009, 603651. [Google Scholar] [CrossRef]
- Hamrani, O.; Hank, Z. Studies on Anti-Inflammatory, Antioxidant and Free Radical Scavenging of Cu (Ii)-N-Acetyl-Para-Aminophenol (APAP) Based Complex. J. Glob. Pharma Technol. 2019, 11, 268–279. [Google Scholar]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Ruiz-Azuara, L.; Bravo-Gomez, M.E. Copper Compounds in Cancer Chemotherapy. Curr. Med. Chem. 2010, 17, 3606–3615. [Google Scholar] [CrossRef]
- Figueroa-DePaz, Y.; Pérez-Villanueva, J.; Soria-Arteche, O.; Martínez-Otero, D.; Gómez-Vidales, V.; Ortiz-Frade, L.; Ruiz-Azuara, L. Casiopeinas of Third Generations: Synthesis, Characterization, Cytotoxic Activity and Structure–Activity Relationships of Mixed Chelate Compounds with Bioactive Secondary Ligands. Molecules 2022, 27, 3504. [Google Scholar] [CrossRef]
- Aguilar-Jiménez, Z.; González-Ballesteros, M.; Dávila-Manzanilla, S.G.; Espinoza-Guillén, A.; Ruiz-Azuara, L. Development and In Vitro and In Vivo Evaluation of an Antineoplastic Copper(II) Compound (Casiopeina III-Ia) Loaded in Nonionic Vesicles Using Quality by Design. Int. J. Mol. Sci. 2022, 23, 12756. [Google Scholar] [CrossRef]
- Aguilar-Jiménez, Z.; Espinoza-Guillén, A.; Resendiz-Acevedo, K.; Fuentes-Noriega, I.; Mejía, C.; Ruiz-Azuara, L. The Importance of Being Casiopeina as Polypharmacologycal Profile (Mixed Chelate–Copper (II) Complexes and Their In Vitro and In Vivo Activities). Inorganics 2023, 11, 394. [Google Scholar] [CrossRef]
- Serment-Guerrero, J.; Bravo-Gomez, M.E.; Lara-Rivera, E.; Ruiz-Azuara, L. Genotoxic Assessment of the Copper Chelated Compounds Casiopeinas: Clues about Their Mechanisms of Action. J. Inorg. Biochem. 2017, 166, 68–75. [Google Scholar] [CrossRef]
- Yakoumis, I.; Panou, M.; Moschovi, A.M.; Panias, D. Recovery of Platinum Group Metals from Spent Automotive Catalysts: A Review. Clean. Eng. Technol. 2021, 3, 100112. [Google Scholar] [CrossRef]
- Icsel, C.; Yilmaz, V.T.; Cevatemre, B.; Aygun, M.; Ulukaya, E. Structures and Anticancer Activity of Chlorido Platinum(II) Saccharinate Complexes with Mono- and Dialkylphenylphosphines. J. Inorg. Biochem. 2019, 195, 39–50. [Google Scholar] [CrossRef]
- Hyeraci, M.; Colalillo, M.; Labella, L.; Marchetti, F.; Samaritani, S.; Scalcon, V.; Rigobello, M.P.; Dalla Via, L. Platinum(II) Complexes Bearing Triphenylphosphine and Chelating Oximes: Antiproliferative Effect and Biological Profile in Resistant Cells. ChemMedChem 2020, 15, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, T.; Wang, Y.; Wang, Z.; Zhu, Z.; Guo, Z.; Wang, X. DNA Topoisomerases as Additional Targets for Anticancer Monofunctional Platinum(II) Complexes. Dalton Trans. 2021, 50, 304–310. [Google Scholar] [CrossRef]
- Kashif Amir, M.; Hogarth, G.; Khan, Z.; Imran, M. Platinum(II) Dithiocarbamate Complexes [Pt(S2CNR2)Cl(PAr3)] as Anticancer and DNA-Damaging Agents. Inorganica Chim. Acta 2020, 512, 119853. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, C.; He, Y.; Zhang, Z.; Zhou, W.; Muhammad, N.; Guo, Y.; Wang, X.; Guo, Z. Restraining Cancer Cells by Dual Metabolic Inhibition with a Mitochondrion-Targeted Platinum(II) Complex. Angew. Chem. Int. Ed. 2019, 58, 4638–4643. [Google Scholar] [CrossRef]
- Muhammad, N.; Tan, C.-P.; Muhammad, K.; Wang, J.; Sadia, N.; Pan, Z.-Y.; Ji, L.-N.; Mao, Z.-W. Mitochondria-Targeting Monofunctional Platinum(ii)–Lonidamine Conjugates for Cancer Cell de-Energization. Inorg. Chem. Front. 2020, 7, 4010–4019. [Google Scholar] [CrossRef]
- Yilmaz, V.T.; Icsel, C.; Turgut, O.R.; Aygun, M.; Erkisa, M.; Turkdemir, M.H.; Ulukaya, E. Synthesis, Structures and Anticancer Potentials of Platinum(II) Saccharinate Complexes of Tertiary Phosphines with Phenyl and Cyclohexyl Groups Targeting Mitochondria and DNA. Eur. J. Med. Chem. 2018, 155, 609–622. [Google Scholar] [CrossRef]
- Li, J.; He, X.; Zou, Y.; Chen, D.; Yang, L.; Rao, J.; Chen, H.; Chan, M.C.W.; Li, L.; Guo, Z.; et al. Mitochondria-Targeted Platinum(ii) Complexes: Dual Inhibitory Activities on Tumor Cell Proliferation and Migration/Invasion via Intracellular Trafficking of β-Catenin. Metallomics 2017, 9, 726–733. [Google Scholar] [CrossRef]
- Guo, Z.; Tong, W.-L.; Chan, M.C.W. Luminescent Oligo(Ethylene Glycol)-Functionalized Cyclometalated Platinum(Ii) Complexes: Cellular Characterization and Mitochondria-Specific Localization. Chem. Commun. 2014, 50, 1711. [Google Scholar] [CrossRef]
- Qin, Q.-P.; Wang, S.-L.; Tan, M.-X.; Wang, Z.-F.; Luo, D.-M.; Zou, B.-Q.; Liu, Y.-C.; Yao, P.-F.; Liang, H. Novel Tacrine Platinum(II) Complexes Display High Anticancer Activity via Inhibition of Telomerase Activity, Dysfunction of Mitochondria, and Activation of the P53 Signaling Pathway. Eur. J. Med. Chem. 2018, 158, 106–122. [Google Scholar] [CrossRef]
- Jany, T.; Moreth, A.; Gruschka, C.; Sischka, A.; Spiering, A.; Dieding, M.; Wang, Y.; Samo, S.H.; Stammler, A.; Bögge, H.; et al. Rational Design of a Cytotoxic Dinuclear Cu2 Complex That Binds by Molecular Recognition at Two Neighboring Phosphates of the DNA Backbone. Inorg. Chem. 2015, 54, 2679–2690. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Dixit, A.; Mitra, K.; Banerjee, S.; Karande, A.A.; Chakravarty, A.R. BODIPY Appended Copper(ii) Complexes of Curcumin Showing Mitochondria Targeted Remarkable Photocytotoxicity in Visible Light. Med. Chem. Commun. 2015, 6, 846–851. [Google Scholar] [CrossRef]
- Gu, S.; Yu, P.; Hu, J.; Liu, Y.; Li, Z.; Qian, Y.; Wang, Y.; Gou, Y.; Yang, F. Mitochondria-Localizing N-Heterocyclic Thiosemicarbazone Copper Complexes with Good Cytotoxicity and High Antimetastatic Activity. Eur. J. Med. Chem. 2019, 164, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yao, Q.; Tian, L.; Wang, Y. Piperidylthiosemicarbazones Cu(II) Complexes with a High Anticancer Activity by Catalyzing Hydrogen Peroxide to Degrade DNA and Promote Apoptosis. Eur. J. Med. Chem. 2018, 158, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ni, H.; Liu, F.; Gu, S.; Yu, P.; Gou, Y. Binuclear Schiff Base Copper(II) Complexes: Syntheses, Crystal Structures, HSA Interaction and Anti-Cancer Properties. Inorganica Chim. Acta 2020, 499, 119186. [Google Scholar] [CrossRef]
- Vutey, V.; Castelli, S.; D’Annessa, I.; Sâmia, L.B.P.; Souza-Fagundes, E.M.; Beraldo, H.; Desideri, A. Human Topoisomerase IB Is a Target of a Thiosemicarbazone Copper(II) Complex. Arch. Biochem. Biophys. 2016, 606, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.G.; Recio Despaigne, A.A.; Louro, S.R.W.; Bandeira, C.C.; Souza-Fagundes, E.M.; Beraldo, H. Cytotoxic Activity, Albumin and DNA Binding of New Copper(II) Complexes with Chalcone-Derived Thiosemicarbazones. Eur. J. Med. Chem. 2013, 65, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.; De Franco, M.; Kellett, A.; Dempsey, E.; Marzano, C.; Erxleben, A.; Gandin, V.; Montagner, D. Anticancer Activity, DNA Binding and Cell Mechanistic Studies of Estrogen-Functionalised Cu(II) Complexes. J. Biol. Inorg. Chem. 2020, 25, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Bielenica, A.; Drzewiecka-Antonik, A.; Rejmak, P.; Stefańska, J.; Koliński, M.; Kmiecik, S.; Lesyng, B.; Włodarczyk, M.; Pietrzyk, P.; Struga, M. Synthesis, Structural and Antimicrobial Studies of Type II Topoisomerase-Targeted Copper(II) Complexes of 1,3-Disubstituted Thiourea Ligands. J. Inorg. Biochem. 2018, 182, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaiah, M.K.; Mokkandi Palsamy, K.; Lokesh, R.; Gandhi, N.I.; Mitu, L.; Jegathalaprathaban, R.; Gurusamy, R. Ternary Copper (II) Complex Based Chemical Probes for DNA Targeting: Cytotoxic Activity under Visible Light. Appl. Organom Chemis 2018, 33, e4762. [Google Scholar] [CrossRef]
- Li, M.; Shao, J.; Guo, Z.; Jin, C.; Wang, L.; Wang, F.; Jia, Y.; Zhu, Z.; Zhang, Z.; Zhang, F.; et al. Novel Mitochondrion-targeting Copper(II) Complex Induces HK2 Malfunction and Inhibits Glycolysis via Drp1-mediating Mitophagy in HCC. J. Cell. Mol. Med. 2020, 24, 3091–3107. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Li, M.; Guo, Z.; Jin, C.; Zhang, F.; Ou, C.; Xie, Y.; Tan, S.; Wang, Z.; Zheng, S.; et al. TPP-Related Mitochondrial Targeting Copper (II) Complex Induces P53-Dependent Apoptosis in Hepatoma Cells through ROS-Mediated Activation of Drp1. Cell Commun. Signal 2019, 17, 149. [Google Scholar] [CrossRef]
- Qin, Q.-P.; Zou, B.-Q. Tryptanthrin Derivatives Copper(II) Complexes with High Antitumor Activity by Inhibiting Telomerase Activity, and Inducing Mitochondria-Mediated Apoptosis and S-Phase Arrest in BEL-7402. New J. Chem. 2018, 42, 15479. [Google Scholar] [CrossRef]
- Shi, X.; Fang, H.; Guo, Y.; Yuan, H.; Guo, Z.; Wang, X. Anticancer Copper Complex with Nucleus, Mitochondrion and Cyclooxygenase-2 as Multiple Targets. J. Inorg. Biochem. 2019, 190, 38–44. [Google Scholar] [CrossRef]
- Davila-Manzanilla, S.G.; Figueroa-de-Paz, Y.; Mejia, C.; Ruiz-Azuara, L. Synergistic Effects between a Copper-Based Metal Casiopeína III-Ia and Cisplatin. Eur. J. Med. Chem. 2017, 129, 266–274. [Google Scholar] [CrossRef]
- Alshater, H.; Al-Sulami, A.I.; Aly, S.A.; Abdalla, E.M.; Sakr, M.A.; Hassan, S.S. Antitumor and Antibacterial Activity of Ni(II), Cu(II), Ag(I), and Hg(II) Complexes with Ligand Derived from Thiosemicarbazones: Characterization and Theoretical Studies. Molecules 2023, 28, 2590. [Google Scholar] [CrossRef]
- Aly, S.A.; Hassan, S.S.; El-Boraey, H.A.; Eldourghamy, A.; Abdalla, E.M.; Alminderej, F.M.; Elganzory, H.H. Synthesis, Biological Activity, and the Effect of Ionization Radiation on the Spectral, XRD, and TGA Analysis of Cu(I), Cu(II), Zn(II), and Cd(II) Complexes. Arab. J. Sci. Eng. 2024, 49, 361–379. [Google Scholar] [CrossRef]
- Amin, A.; Buratovich, M. New Platinum and Ruthenium Complexes—The Latest Class of Potential Chemotherapeutic Drugs—A Review of Recent Developments in the Field. MRMC 2009, 9, 1489–1503. [Google Scholar] [CrossRef] [PubMed]
- Deo, K.; Pages, B.; Ang, D.; Gordon, C.; Aldrich-Wright, J. Transition Metal Intercalators as Anticancer Agents—Recent Advances. Int. J. Mol. Sci. 2016, 17, 1818. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper as a Biocidal Tool. Curr. Med. Chem. 2005, 12, 2163–2175. [Google Scholar] [CrossRef]
- Lisic, E.C.; Grossarth, S.N.; Bowman, S.B.; Hill, J.L.; Beck, M.W.; Deweese, J.E.; Jiang, X. New Copper (II), Palladium (II), and Platinum (II) 2-Acetylpyrazine Tert-Butylthiosemicarbazone Complexes: Inhibition of Human Topoisomerase IIα and Activity against Breast Cancer Cells. Open J. Med. Chem. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- May, N.V.; Jancsó, A.; Enyedy, É.A. Binding Models of Copper(II) Thiosemicarbazone Complexes with Human Serum Albumin: A Speciation Study. Molecules 2021, 26, 2711. [Google Scholar] [CrossRef]
- Summers, K.L.; Roseman, G.P.; Sopasis, G.J.; Millhauser, G.L.; Harris, H.H.; Pickering, I.J.; George, G.N. Copper(II) Binding to PBT2 Differs from That of Other 8-Hydroxyquinoline Chelators: Implications for the Treatment of Neurodegenerative Protein Misfolding Diseases. Inorg. Chem. 2020, 59, 17519–17534. [Google Scholar] [CrossRef]
- Szymański, P.; Frączek, T.; Markowicz, M.; Mikiciuk-Olasik, E. Development of Copper Based Drugs, Radiopharmaceuticals and Medical Materials. Biometals 2012, 25, 1089–1112. [Google Scholar] [CrossRef]
- Yadav, A.A.; Patel, D.; Wu, X.; Hasinoff, B.B. Molecular Mechanisms of the Biological Activity of the Anticancer Drug Elesclomol and Its Complexes with Cu(II), Ni(II) and Pt(II). J. Inorg. Biochem. 2013, 126, 1–6. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Yan, M.; Wang, H.; Zhang, C. Novel Copper Complexes as Potential Proteasome Inhibitors for Cancer Treatment. Mol. Med. Rep. 2017, 15, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Almaqwashi, A.A.; Paramanathan, T.; Rouzina, I.; Williams, M.C. Mechanisms of Small Molecule–DNA Interactions Probed by Single-Molecule Force Spectroscopy. Nucleic Acids Res. 2016, 44, 3971–3988. [Google Scholar] [CrossRef] [PubMed]
- Andrezálová, L.; Országhová, Z. Covalent and Noncovalent Interactions of Coordination Compounds with DNA: An Overview. J. Inorg. Biochem. 2021, 225, 111624. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, Z.; Zhang, C.; Wang, Y.; Zhang, H.; Gan, Z.; Guo, Z.; Wang, X. Mitochondrion-Targeted Platinum Complexes Suppressing Lung Cancer through Multiple Pathways Involving Energy Metabolism. Chem. Sci. 2019, 10, 3089–3095. [Google Scholar] [CrossRef]






| Formula | Targets of Action | Reference |
|---|---|---|
| Platinum(II) | ||
| C23H26ClNO3P2PtS | DNA/Mitochondria | [48] |
| C25H21ClNO2PPt·H2O | DNA/Mitochondria | [49] |
| (C24H27ClN3PPt)(NO3)2 | DNA/Mitochondria/Topoisomerase | [50] |
| C44H41Cl4N16O4Pt2S4·6DMSO | DNA/PTP1B | [23] |
| C26H25ClN2O3Pt·H2O | Mitochondria | [17,20] |
| C22H14Cl2N4Pt | DNA | [18] |
| C18H17ClN4OPt | HSA | [21] |
| (C46H36N6O2Pt2I2)2+ | DNA/Topoisomerase | [14] |
| C31H33ClN3PPtS2 | DNA | [22] |
| C29H25ClF3N2OPPtS2 | DNA | [51] |
| C14H9Cl3N2OPt | Mitochondria | [15] |
| C40H31ClN3PPt | Mitochondria | [52] |
| C16H9Cl2FN2OPtS | Mitochondria | [16] |
| C45H37Cl3N7OPt·NO3 | Mitochondria | [53] |
| C50H50N2O6P2PtS2 | DNA/Mitochondria | [54] |
| C53H55N3O4PPt·ClO4 | Mitochondria | [55,56] |
| C42H29N6Pt·Cl | Mitochondria/Telomerase | [57] |
| Copper(II) | ||
| C44H46N6Cu2O6·9.75H2O | DNA | [58] |
| C53H49BCuF2I2N5O6·Cl | Mitochondria | [59] |
| C26H26Br3Cu3N8S2 | Mitochondria | [60] |
| (C17H18BrCuN5S)2 | AND/Mitochondria | [61] |
| C29H24Cu2F2N6O11 | Mitochondria | [62] |
| C15H12BrClCuN4·DMF | Topoisomerase/BSA | [63,64] |
| C54H42CuN6O2·(NO2)2 | DNA | [65] |
| Cu(C14H8Cl2F3N2S)2·3H2O·0.5DMF | Topoisomerase | [66] |
| C26H17BrClCuN5O1S·C3H6O | DNA/HSA | [67] |
| (C40H31Br2CuN3P)Br | Mitochondria | [68,69] |
| C30H16Cl2CuN4O4 | DNA/Mitochondria/Telomerases | [70] |
| C39H39CuN3O9 | DNA/Mitochondria/COX-2 | [71] |
| C17H19CuN2O2·H2O·NO3 | DNA | [72] |
| C16H16CuN3O2·2H2O·NO3 | DNA | [72] |
| C30H29Cl2CuN8O2S2 | 3GEY/1m17 | [73] |
| C32H36N8O10S2Cl2Cu | Topoisomerase | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciel-Flores, C.E.; Lozano-Alvarez, J.A.; Bivián-Castro, E.Y. Recently Reported Biological Activities and Action Targets of Pt(II)- and Cu(II)-Based Complexes. Molecules 2024, 29, 1066. https://doi.org/10.3390/molecules29051066
Maciel-Flores CE, Lozano-Alvarez JA, Bivián-Castro EY. Recently Reported Biological Activities and Action Targets of Pt(II)- and Cu(II)-Based Complexes. Molecules. 2024; 29(5):1066. https://doi.org/10.3390/molecules29051066
Chicago/Turabian StyleMaciel-Flores, Cristhian Eduardo, Juan Antonio Lozano-Alvarez, and Egla Yareth Bivián-Castro. 2024. "Recently Reported Biological Activities and Action Targets of Pt(II)- and Cu(II)-Based Complexes" Molecules 29, no. 5: 1066. https://doi.org/10.3390/molecules29051066
APA StyleMaciel-Flores, C. E., Lozano-Alvarez, J. A., & Bivián-Castro, E. Y. (2024). Recently Reported Biological Activities and Action Targets of Pt(II)- and Cu(II)-Based Complexes. Molecules, 29(5), 1066. https://doi.org/10.3390/molecules29051066