Effect of Copper Antifouling Paint on Marine Degradation of Polypropylene: Uneven Distribution of Microdebris between Nagasaki Port and Goto Island, Japan
Abstract
:1. Introduction
2. Results and Discussion
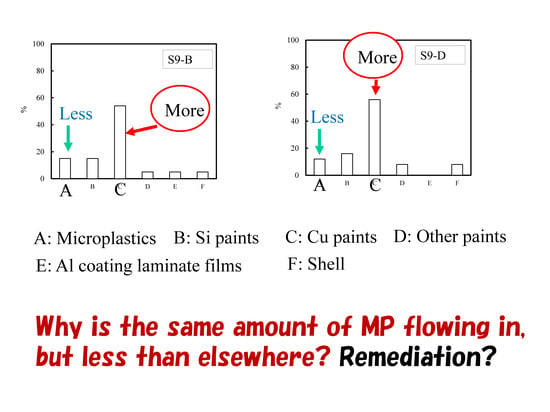
2.1. Types of Marine Tiny Particles and Their Distribution Behavior
2.2. Ionization Behavior of Cuprous Oxide in Seawater and Its Effect on PP Degradation
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.2.1. Scanning Electron Microscope (SEM) with Energy-Dispersive X-ray Spectroscopy Analysis
3.2.2. Fourier Transform Infrared (FT-IR) Analysis
3.2.3. Gel Permeation Chromatography (GPC) Analysis
3.3. Methods
3.3.1. MP Sampling from the Sea
3.3.2. Filtration for SEM/EDX Observation of MP Retrieved from the Sea
3.3.3. Preparation of PP Sample Containing Cuprous Oxide (Cu2O)
3.4. Enhanced Degradation Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Poll. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Thompson, R.C.; Swan, S.H.; Moore, C.J.; vom Saal, F.S. Our plastic age. Phil. Trans. R. Soc. B 2009, 364, 1973–1976. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Halle, A.T.; Ladirat, L.; Gendre, X.; Goudouneche, D.; Pusineri, C.; Routaboul, C.; Tenailleau, C.; Duployer, B.; Perez, E. Understanding the fragmentation pattern of marine plastic debris. Environ. Sci. Technol. 2016, 50, 5668–5675. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11. [Google Scholar] [CrossRef]
- Yokota, K.; Waterfield, H.; Hastings, C.; Davidson, E.; Kwietniewski, E.; Wells, B. Finding the missing piece of the aquatic plastic pollution puzzle: Interaction between primary producers and microplastics. Limnol. Oceanogr. Lett. 2017, 2, 91–104. [Google Scholar] [CrossRef]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Farrell, P.; Nelson, K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut. 2013, 177, 1–3. [Google Scholar] [CrossRef]
- Pellini, G.; Gomiero, A.; Fortibuoni, T.; Ferrà, C.; Grati, F.; Tassetti, A.N.; Polidori, P.; Fabi, G.; Scarcella, G. Characterization of microplastic litter in the gastrointestal tract of Solea solea from the Adriatic Sea. Environ. Pollut. 2018, 234, 943–952. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Formation of microscopic particles during the degradation of different polymers. Chemosphere 2016, 161, 510–517. [Google Scholar] [CrossRef]
- Julienne, F.; Delorme, N.; Lagarde, F. From macroplastics to microplastics: Role of water in the fragmentation of polyethylene. Chemosphere 2019, 236, 124409. [Google Scholar] [CrossRef]
- Julienne, F.; Lagarde, F.; Delorme, N. Influence of the crystalline structure on the fragmentation of weathered polyolefines. Polym. Degrad. Stab. 2019, 170, 109012. [Google Scholar] [CrossRef]
- Nakatani, H.; Ohshima, Y.; Uchiyama, T.; Motokucho, S.; Dao, A.T.N.; Kim, H.J.; Yagi, M.; Kyozuka, Y. Rapid oxidative fragmentation of polypropylene with pH control in seawater for preparation of realistic reference microplastics. Sci. Rep. 2023, 13, 4247. [Google Scholar] [CrossRef]
- Almeida, E.; Diamantino, T.C.; Sousa, O.D. Marine paints: The particular case of antifouling paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Singh, N.; Turner, A. Trace metals in antifouling paint particles and their heterogeneous contamination of coastal sediments. Mar. Pollut. Bull. 2009, 58, 559–564. [Google Scholar] [CrossRef]
- Ito, N.; Eriguchi, T.; Ichikawa, T.; Horiguchi, F. Methodological study on ways to reduce ecological risk posed to Tokyo Bay by antifouling paints containing copper pyrithione. J. JIME 2009, 44, 85–91. [Google Scholar] [CrossRef]
- Singh, N.; Turner, A. Leaching of copper and zinc from spent antifouling paint particles. Environ. Pollut. 2009, 157, 371–376. [Google Scholar] [CrossRef]
- Isobe, A.; Iwasaki, S.; Uchida, K.; Tokai, T. Abundance of non-conservative microplastics in the upper ocean from 1957 to 2066. Nat. Commun. 2019, 10, 417. [Google Scholar] [CrossRef]
- Dale, E.N.; Nicholas, W.M.R. Quantitative SEM/EDS, step 1: What constitutes a sufficiently flat specimen? Microsc. Microanal. 2013, 19, 1244–1245. [Google Scholar]
- Chan, M.G.; Allara, D.L. Infrared reflection studies of the mechanism of oxidation at a copper—Polyethylene interface. J. Colloid. Interface Sci. 1974, 47, 697–704. [Google Scholar] [CrossRef]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef]
- Furukawa Chemicals Co., Ltd. Cuprous Oxide. Available online: https://www.furukawachemicals.co.jp/cu2o.html (accessed on 21 September 2023).
- Nakatani, H.; Muraoka, T.; Ohshima, Y.; Motokucho, S. Difference in polypropylene fragmentation mechanism between marine and terrestrial regions. SN Appl. Sci. 2021, 3, 773. [Google Scholar] [CrossRef]
- Nakatani, H.; Ohshima, Y.; Uchiyama, T.; Motokucho, S. Degradation and fragmentation behavior of polypropylene and polystyrene in water. Sci. Rep. 2022, 12, 18501. [Google Scholar] [CrossRef]
- Mikdam, A.; Colina, X.; Minard, G.; Billon, N.; Maurin, R. A kinetic model for predicting the oxidative degradation of additive free polyethylene in bleach desinfected water. Polym. Degrad. Stab. 2017, 146, 76–94. [Google Scholar] [CrossRef]
- Ito, S.; Ukawa, T.; Kishimoto, N.; Kato, M.; Otsu, H. Technical feasibility of electrochemical fenton-type process using Cu(I)/HOCl system. J. Water Environ. Technol. 2018, 16, 73–82. [Google Scholar] [CrossRef]
- Poling, G.W.J. Infrared reflection studies of the oxidation of copper and iron. J. Electrochem. Soc. 1969, 116, 958–963. [Google Scholar] [CrossRef]
- Howell, D.; Behrends, B. A methodology for evaluating biocide release rate, surface roughness and leach layer formation in a TBT-free, self-polishing antifouling formulation. Biofouling 2006, 22, 303–315. [Google Scholar] [CrossRef]
- Gugumus, F. Re-examination of the role of hydroperoxides in polyethylene and polypropylene: Chemical and physical aspects of hydroperoxides in polyethylene. Polym. Degrad. Stab. 1995, 49, 29–50. [Google Scholar] [CrossRef]
- Gugumus, F. Thermooxidative degradation of polyolefins in the solid state-6. Kinetics of thermal oxidation of polypropylene. Polym. Degrad. Stab. 1998, 62, 235–243. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Peana, M.; Bekiari, V. Structural identification of metalloproteomes in marine diatoms, an efficient algae model in toxic metals bioremediation. Molecules 2022, 27, 378. [Google Scholar] [CrossRef]
- Kumar, R.; Paturzo, M.; Sardo, A.; Orefice, I.; Yu, Q.; Rubano, A.; Paparo, D. Toxic effect of metal doping on diatoms as probed by broadband terahertz time-domain spectroscopy. Molecules 2022, 27, 5897. [Google Scholar] [CrossRef]
- Nedyalkova, M.; Simeonov, V. Developing an intelligent data analysis approach for marine sediments. Molecules 2022, 27, 6539. [Google Scholar] [CrossRef]
- Carlsson, D.J.; Wiles, D.M. The photodegradation of polypropylene films. III. photolysis of polypropylene hydroperoxides. Macromolecules 1969, 6, 597–606. [Google Scholar] [CrossRef]
- Kato, Y.; Carlsson, D.J.; Wiles, D.M. The photo-oxidation of polypropylene: Some effects of molecular order. J. Appl. Polym. Sci. 1969, 13, 1447–1458. [Google Scholar] [CrossRef]
- Canopoli, L.; Coulon, F.; Wagland, S.T. Degradation of excavated polyethylene and polypropylene waste from landfill. Sci. Total. Environ. 2020, 698, 134125. [Google Scholar] [CrossRef]
- Yagi, M.; Kobayashi, T.; Maruyama, Y.; Hoshina, S.; Masumi, S.; Aizawa, I.; Uchida, J.; Kinoshita, T.; Yamawaki, N.; Aoshima, T.; et al. Microplastic pollution of commercial fishes from coastal and offshore waters in southwestern Japan. Mar. Pollut. Bull. 2022, 174, 113304. [Google Scholar] [CrossRef]









| Samples | Deg. Days | Mn | Mw | Mz | Mw/Mn | Mz/Mw |
|---|---|---|---|---|---|---|
| PP original | 0 | 50,120 | 286,262 | 953,225 | 5.7 | 3.3 |
| Degraded PP in normal seawater | 3 | 25,792 | 109,773 | 263,810 | 4.3 | 2.4 |
| 9 | 9438 | 30,076 | 65,159 | 3.2 | 2.2 | |
| 15 | 4727 | 9950 | 18,526 | 2.1 | 1.9 | |
| Degraded PP containing 5 phr Cu2O in normal seawater | 3 | 18,044 | 76,769 | 187,614 | 4.3 | 2.4 |
| 9 | 6187 | 15,258 | 30,579 | 2.5 | 2.0 | |
| 15 | 4582 | 9589 | 17,767 | 2.1 | 1.9 | |
| Degraded PP containing 5 phr Cu2O in three times salinity conc. seawater | 3 | 18,374 | 71,490 | 163,820 | 3.9 | 2.3 |
| 9 | 7282 | 19,889 | 40,817 | 2.7 | 2.1 | |
| 15 | 4935 | 10,256 | 18,686 | 2.1 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakatani, H.; Yamashiro, K.; Uchiyama, T.; Motokucho, S.; Dao, A.T.N.; Kim, H.-J.; Yagi, M.; Kyozuka, Y. Effect of Copper Antifouling Paint on Marine Degradation of Polypropylene: Uneven Distribution of Microdebris between Nagasaki Port and Goto Island, Japan. Molecules 2024, 29, 1173. https://doi.org/10.3390/molecules29051173
Nakatani H, Yamashiro K, Uchiyama T, Motokucho S, Dao ATN, Kim H-J, Yagi M, Kyozuka Y. Effect of Copper Antifouling Paint on Marine Degradation of Polypropylene: Uneven Distribution of Microdebris between Nagasaki Port and Goto Island, Japan. Molecules. 2024; 29(5):1173. https://doi.org/10.3390/molecules29051173
Chicago/Turabian StyleNakatani, Hisayuki, Kaito Yamashiro, Taishi Uchiyama, Suguru Motokucho, Anh Thi Ngoc Dao, Hee-Jin Kim, Mitsuharu Yagi, and Yusaku Kyozuka. 2024. "Effect of Copper Antifouling Paint on Marine Degradation of Polypropylene: Uneven Distribution of Microdebris between Nagasaki Port and Goto Island, Japan" Molecules 29, no. 5: 1173. https://doi.org/10.3390/molecules29051173
APA StyleNakatani, H., Yamashiro, K., Uchiyama, T., Motokucho, S., Dao, A. T. N., Kim, H. -J., Yagi, M., & Kyozuka, Y. (2024). Effect of Copper Antifouling Paint on Marine Degradation of Polypropylene: Uneven Distribution of Microdebris between Nagasaki Port and Goto Island, Japan. Molecules, 29(5), 1173. https://doi.org/10.3390/molecules29051173