Advances in Understanding the Antioxidant and Antigenic Properties of Egg-Derived Peptides
Abstract
:1. Introduction
2. Results
2.1. Influence of the Enzyme on Egg Protein Hydrolysis
2.2. SDS-PAGE Analysis of Egg Protein Hydrolysates

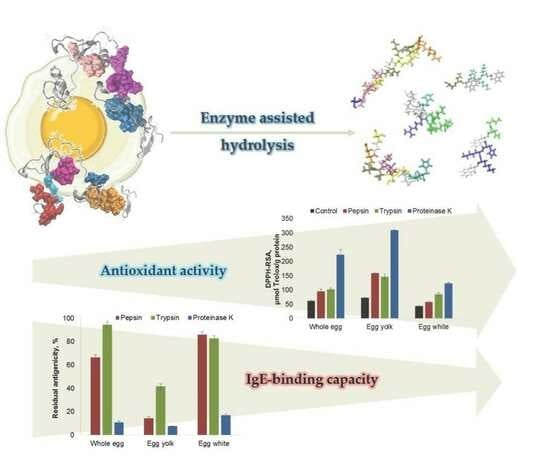
2.3. Influence of Enzyme-Assisted Hydrolysis on the Antioxidant Activity of the Egg Proteins
2.4. Influence of Protein Hydrolysis on the Antigenic Properties of Egg and Egg Derivatives
2.5. In Silico Observations of the Main Egg Allergens

3. Materials and Methods
3.1. Materials
3.2. Obtaining the Egg Protein Hydrolysates
3.3. Determination of the Degree of Hydrolysis
3.4. SDS-PAGE Analysis
3.5. Antioxidant Activity
3.6. Assessment of the Bioactive Peptides with Antioxidant Activity
3.7. IgE-Binding Properties
3.8. In Silico Investigations on the Main Egg Allergens
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The golden egg: Nutritional value, bioactivities, and emerging benefits for human health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef]
- Sarantidi, E.; Ainatzoglou, A.; Papadimitriou, C.; Stamoula, E.; Maghiorou, K.; Miflidi, A.; Trichopoulou, A.; Mountzouris, K.C.; Anagnostopoulos, A.K. Egg White and Yolk Protein Atlas: New Protein Insights of a Global Landmark Food. Foods 2023, 12, 3470. [Google Scholar] [CrossRef]
- ANSES-CIQUAL Food Composition Table. 2020. Available online: https://www.anses.fr/en/content/anses-ciqual-food-composition-table (accessed on 29 January 2024).
- Chang, C.; Lahti, T.; Tanaka, T.; Nickerson, M.T. Egg proteins: Fractionation, bioactive peptides and allergenicity. J. Sci. Food Agric. 2018, 98, 5547–5558. [Google Scholar] [CrossRef]
- Ma, X.; Liang, R.; Xing, Q.; Lozano-Ojalvo, D. Can food processing produce hypoallergenic egg? J. Food Sci. 2020, 85, 2635–2644. [Google Scholar] [CrossRef]
- Afraz, M.T.; Khan, M.R.; Roobab, U.; Noranizan, M.A.; Tiwari, B.K.; Rashid, M.T.; Inam-ur-Raheem, M.I.; Aadil, R.M. Impact of novel processing techniques on the functional properties of egg products and derivatives: A review. J. Food Process Eng. 2020, 43, e13568. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Bekhit, A.E.D.A.; Kumar, S.; Bhat, H.F. Effect of processing technologies on the digestibility of egg proteins. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4703–4738. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; D’Silva, I. Bioactive components in egg white. In Egg Bioscience and Biotechnology; Mine, Y., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 141–184. [Google Scholar]
- Dona, D.W.; Suphioglu, C. Egg allergy: Diagnosis and immunotherapy. Int. J. Mol. Sci. 2020, 21, 5010. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Yang, M. Recent advances in the understanding of egg allergens: Basic, industrial, and clinical perspectives. J. Agric. Food Chem. 2008, 56, 4874–4900. [Google Scholar] [CrossRef] [PubMed]
- Stănciuc, N.; Banu, I.; Turturică, M.; Aprodu, I. pH and heat induced structural changes of chicken ovalbumin in relation with antigenic properties. Int. J. Biol. Macromol. 2016, 93, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Tavano, O.L. Protein hydrolysis using proteases: An important tool for food biotechnology. J. Mol. Catal. B Enzym. 2013, 90, 1–11. [Google Scholar] [CrossRef]
- Huhmann, S.; Stegemann, A.K.; Folmert, K.; Klemczak, D.; Moschner, J.; Kube, M.; Koksch, B. Position-dependent impact of hexafluoroleucine and trifluoroisoleucine on protease digestion. Beilstein J. Org. Chem. 2017, 13, 2869–2882. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Oey, I.; Bremer, P.; Silcock, P.; Carne, A. Proteolytic pattern, protein breakdown and peptide production of ovomucin-depleted egg white processed with heat or pulsed electric fields at different pH. Food Res. Int. 2018, 108, 465–474. [Google Scholar] [CrossRef]
- Saxena, I.; Tayyab, S. Protein proteinase inhibitors from avian egg whites. Cell Mol. Life Sci. 1997, 53, 13–23. [Google Scholar] [CrossRef]
- Koç, M.; Koç, B.; Susyal, G.; Sakin Yilmazer, M.; Kaymak Ertekin, F.; Bağdatlıoğlu, N. Functional and physicochemical properties of whole egg powder: Effect of spray drying conditions. J. Food Sci. Technol. 2011, 48, 141–149. [Google Scholar] [CrossRef]
- Lechevalier, V.; Guérin-Dubiard, C.; Anton, M.; Beaumal, V.; Briand, E.D.; Gillard, A.; Le Gouar, Y.; Musikaphun, N.; Tanguy, G.; Pasco, M.; et al. Pasteurisation of liquid whole egg: Optimal heat treatments in relation to its functional, nutritional and allergenic properties. J. Food Eng. 2017, 195, 137–149. [Google Scholar] [CrossRef]
- Yoshino, K.; Sakai, K.; Mizuha, Y.; Shimizuike, A.; Yamamoto, S. Peptic digestibility of raw and heat-coagulated hen’s egg white proteins at acidic pH range. Int. J. Food Sci. Nutr. 2004, 55, 635–640. [Google Scholar] [CrossRef]
- Van der Plancken, I.; Van Remoortere, M.; Van Loey, A.; Hendrickx, M.E. Trypsin inhibition activity of heat-denatured ovomucoid: A kinetic study. Biocatal. Bioreact. Des. 2004, 20, 82–86. [Google Scholar] [CrossRef]
- McNamara, D.J. Eggs. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 132–138. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, Y.; Jiang, Y.; Chi, Y. Effect of dry heating on the aggregation behaviour and aggregate morphologies of ovalbumin. Food Chem. 2019, 285, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Mann, M. The chicken egg yolk plasma and granule proteomes. Proteomics 2008, 8, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Uysal, R.S.; Acar-Soykut, E.; Boyaci, I.H. Determination of yolk:white ratio of egg using SDS-PAGE. Food Sci. Biotechnol. 2019, 29, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Lassé, M.; Deb-Choudhury, S.; Haines, S.; Larsen, N.; Gerrard, J.A.; Dyer, J.M. The impact of pH, salt concentration and heat on digestibility and amino acid modification in egg white protein. J. Food Compos. Anal. 2015, 38, 42–48. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Jo, C.; Suh, J.W.; Ahn, D.U. Enzymatic hydrolysis of ovomucoid and the functional properties of its hydrolysates. Poult. Sci. 2015, 94, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Benedé, S.; Molina, E. Chicken egg proteins and derived peptides with antioxidant properties. Foods 2020, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Aluko, R.E. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int. J. Mol. Sci. 2011, 12, 3148–3161. [Google Scholar] [CrossRef]
- Xu, N.; Chen, G.; Liu, H. Antioxidative categorization of twenty amino acids based on experimental evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Muhialdin, B.J.; Hassanzadeh, K.; Yea, C.S.; Ahmadi, R. Enzymatic Hydrolysis of Proteins. In Bioactive Peptides from Food—Sources, Analysis, and Functions, 1st ed.; Nollet, L., Ötleş, S., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 189–207. [Google Scholar]
- Dumitrașcu, L.; Lanciu Dorofte, A.; Grigore-Gurgu, L.; Aprodu, I. Proteases as Tools for Modulating the Antioxidant Activity and Functionality of the Spent Brewer’s Yeast Proteins. Molecules 2023, 28, 3763. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Cui, R.; Ji, S.; Xia, M.; Fu, X.; Huang, X. Mechanistic studies of polyphenols reducing the trypsin inhibitory activity of ovomucoid: Structure, conformation, and interactions. Food Chem. 2023, 408, 135063. [Google Scholar] [CrossRef]
- Martos, G.; López-Fandiño, R.; Molina, E. Immunoreactivity of hen egg allergens: Influence on in vitro gastrointestinal digestion of the presence of other egg white proteins and of egg yolk. Food Chem. 2013, 136, 775–781. [Google Scholar] [CrossRef]
- Benedé, S.; López-Expósito, I.; López-Fandiño, R.; Molina, E. Identification of IgE-binding peptides in hen egg ovalbumin digested in vitro with human and simulated gastroduodenal fluids. J. Agric. Food Chem. 2014, 62, 152–158. [Google Scholar] [CrossRef]
- Matsuda, T.; Tsuruta, K.; Nakabe, Y.; Nakamura, R. Reduction of ovomucoid immunogenic activity on peptic fragmentation and heat denaturation. Agric. Biol. Chem. Tokyo 1985, 49, 2237–2241. [Google Scholar] [CrossRef]
- Mine, Y.; Rupa, P. Fine mapping and structural analysis of immunodominant IgE allergenic epitopes in chicken egg ovalbumin. Protein Eng. 2003, 16, 747–752. [Google Scholar] [CrossRef]
- Elsayed, S.; Holen, E.; Haugstad, M.B. Antigenic and allergenic determinants of ovalbumin II. The reactivity of the NH2 terminal decapeptide. Scand. J. Immunol. 1988, 27, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, S.; Stavseng, L. Epitope mapping of region 11-70 of ovalbumin (Gal d I) using five synthetic peptides. Int. Arch. Allergy Immunol. 1994, 104, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, H.; Petersen, A.; Becker, W.M.; Schlaak, M. Epitope analysis of the allergen ovalbumin (Gal d II) with monoclonal antibodies and patients’ IgE. Mol. Immunol. 1992, 29, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Honma, K.; Kohno, Y.; Saito, K.; Shimojo, N.; Horiuchi, T.; Hayashi, H.; Suzuki, N.; Hosoya, T.; Tsunoo, H.; Niimi, H. Allergenic epitopes of ovalbumin (OVA) in patients with hen’s egg allergy: Inhibition of basophil histamine release by haptenic ovalbumin peptide. Clin. Exp. Immunol. 1996, 103, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Botas, J.; Cerecedo, I.; Zamora, J.; Vlaicu, C.; Dieguez, M.C.; Gómez-Coronado, D.; De Dios, V.; Terrados, S.; De La Hoz, B. Mapping of the IgE and IgG4 sequential epitopes of ovomucoid with a peptide microarray immunoassay. Int. Arch. Allergy Immunol. 2013, 161, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.K.; Sampson, H.A. Allergenic properties of ovomucoid in man. J. Immunol. 1997, 159, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Besler, M.; Petersen, A.; Steinhart, H.; Paschke, A. Identification of IgE-binding peptides derived from chemical and enzymatic cleavage of ovomucoid. Internet Symp. Food Allerg. 1999, 1, 1–12. [Google Scholar]
- Holen, E.; Bolann, B.; Elsayed, S. Novel B and T cell epitopes of chicken ovomucoid (Gal d 1) induce T cell secretion of IL-6, IL-13, and IFN-γ. Clin. Exp. Immunol. 2001, 31, 952–964. [Google Scholar] [CrossRef]
- Mine, Y.; Zhang, J.W. Identification and fine mapping of IgG and IgE epitopes in ovomucoid. Biochem. Biophys. Res. Commun. 2002, 292, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, K.M.; Beyer, K.; Vila, L.; Bardina, L.; Mishoe, M.; Sampson, H.A. Specificity of IgE antibodies to sequential epitopes of hen’s egg ovomucoid as a marker for persistence of egg allergy. Allergy 2007, 62, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Salelles, L.; Floury, J.; Le Feunteun, S. Pepsin activity as a function of pH and digestion time on caseins and egg white proteins under static in vitro conditions. Food Funct. 2021, 12, 12468–12478. [Google Scholar] [CrossRef] [PubMed]
- Navarrete del Toro, M.; García-Carreño, F.L. Evaluation of the progress of protein hydrolysis. Curr. Protoc. Food Anal. Chem. 2002, S4, B2.2.1–B2.2.14. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Banu, I.; Vasilean, I.; Aprodu, I. Effect of lactic fermentation on antioxidant capacity of rye sourdough and bread. Food Sci. Technol. Res. 2010, 16, 571–576. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Stein, P.E.; Leslie, A.G.; Finch, J.T.; Carrell, R.W. Crystal structure of uncleaved ovalbumin at 1·95 Å resolution. J. Mol. Biol. 1991, 221, 941–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef] [PubMed]




| Protein Name (UniProt Entry) | Total No. of Encrypted Peptides with Antioxidant Activity | Potential Peptides with Antioxidant Activity Released through Complete Hydrolysis with Various Enzymes | ||
|---|---|---|---|---|
| Pepsin | Trypsin | Proteinase K | ||
| Egg white | ||||
| Ovalbumin (P01012) | 38 | EL | - | EL |
| Ovotransferrin (P02789) | 75 | EL, HL | - | EL, HL, RW, KP, KAI, SGAF |
| Ovomucoid (P01005) | 14 | - | - | KP, TY |
| Lysozyme (P00698) | 15 | - | - | AW |
| Ovomucin α subunit (Q98UI9) | 142 | - | IR | EL, KP, RY, SDF |
| Ovomucin β subunit (F1NBL0) | 76 | EL, HL | - | EL, HL, KKY, QAY |
| Egg yolk | ||||
| Vitellogenin-2 (P02845) | 149 | HL, VKL | IR, LK, AHK, LHR, DYK | EL, HL, AY, KP, RY, TY, AW, KAY |
| Vitellogenin-1 (P87498) | 126 | EL | IR | EL, HL, AY, KP, RY, RW, TY |
| α Livetin (P19121) | 31 | - | - | RY, ADGF |
| Ref. | Amino Acid Sequence [Position in P01012/P01005] | Cleavage Sites | ||
|---|---|---|---|---|
| Pepsin | Trypsin | Proteinase K | ||
| Ovalbumin | ||||
| Mine and Rupa [36] | LAMVYLGAKDST (39–50) | 39 43 44 | 47 | 39 40 42 43 44 46 |
| DVYSFSLA (96–103) | 99 100 101 102 | 105 | 97 98 100 102 | |
| EDTQAMPFRV (192–201) | 198 | 200 | 192 194 196 199 | |
| VLLPDE (244–249) | 244 246 | - | 224 226 230 231 232 233 235 236 239 243 244 245 246 | |
| GLEQLESIIN (252–261) | 252 253 255 256 | - | 253 254 256 257 259 260 | |
| Elsayed et al. [37] | GSIGAASMEF (2–11) | 10 | - | 4 6 7 10 |
| Elsayed and Stavseng [38] | CFDVFKELK (12–20) | 12 13 15 16 18 | - | 13 15 16 18 19 |
| Kahlert et al. [39] | VYLGAKDSTRTQINK VVRFDKLPGFGDSIE AQCGTSVNVHSSLR DILNQITKPNDVYSF SLASRLYAEERYPILP EYLQCVKELYRGGLE PINFQTAADQAREL INSWVESQTNGIIRN VLQPSSVDSQTAM (42–173) | 43 44 60 63 66 84 88 99 100 101 102 106 115 118 119 124 134 135 144 | 47 51 56 59 62 85 105 111 123 127 143 159 | 42 43 44 46 50 52 54 57 58 60 63 66 70 71 72 76 78 80 84 87 88 91 92 97 98 100 102 103 106 107 108 109 110 112 114 115 117 118 119 122 124 125 126 130 131 133 135 137 138 139 142 144 145 146 149 150 151 154 157 158 161 162 167 171 |
| GITDVFSSSANLSGI SSAESLKISQAVHA AHAEINEAGREVV GSAEAGVDAASVS EEFRADHPFLFCIK HIATNAVLFF GRC VSP (302–386) | 306 307 312 313 321 322 358 359 364 366 367 377 378 379 380 | 323 340 360 370 382 | 304 306 307 311 313 316 319 320 322 324 327 328 330 331 333 334 335 337 338 341 342 343 346 347 348 350 352 353 355 357 358 359 361 365 366 367 369 372 373 374 376 377 378 379 380 384 | |
| Honma et al. [40] | EFRADHPFLF (358–367) | 358 359 364 366 | 360 | 358 359 361 365 366 |
| Ovomucoid | ||||
| Cooke and Sampson [42] | AEVDCSRFPNATDKEGKDVL [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] | 32 | 31 38 41 | 25 26 27 32 35 36 39 43 |
| EF (73–74) | 73 | - | 73 | |
| VLCNRAFNPVCG (109–120) | 109 110 114 | 113 | 109 110 114 115 118 | |
| QGASVDKR (139–146) | - | 145 | 141 143 | |
| RPLCGSDNKTYG (175–186) | 176 | - | 177 184 185 | |
| Besler et al. [43] | AFNPVCGTDGVTYDNECLLCAHKVEQGASVDK (114–145) | 114 130 131 132 | 136 145 | 114 115 118 121 124 125 126 129 131 132 134 137 138 141 143 |
| VSVDCSEYPKPDCTAEDRPLCGSDNKTYGNKCNFCNAVVESNGTLTLSHFGKC (158–210) | 176 191 201 202 203 204 206 207 | 183 188 209 | 158 160 164 165 171 172 173 177 184 185 191 194 195 196 197 201 202 203 204 207 | |
| Holen et al. [44] | AEVDCSRFPNATDK (25–38) | 32 | 31 | 25 26 27 32 35 36 |
| ATDKEGKDVLVCNK (35–48) | 32 44 | 38 41 | 35 36 39 43 44 45 | |
| GTDGVTYTNDCLLC (55–68) | 65 66 67 | 56 59 60 61 62 66 67 | ||
| GTNISKEHDGECKE (75–88) | - | 80 87 | 76 78 81 85 | |
| ECKETVPMNCSSYA (85–98) | - | 87 | 85 88 89 90 97 | |
| TYDNECLLCAHKVE (125–138) | 130 131 132 | 136 | 125 126 129 131 132 134 137 | |
| KRHDGGCRKELAAV (145–158) | - | 145 146 153 | 143 154 155 156 157 | |
| Mine and Zhang [45] | TDGVTYTNDCL (56–66) | 65 | - | 56 59 60 61 62 |
| DCLLCAYSIEF (64–74) | 65 66 67 73 | - | 66 67 69 70 72 73 | |
| KEHDGECKETV (80–90) | - | 80 87 | 81 85 88 89 | |
| SSYAN (95–99) | - | - | 97 98 | |
| DGKVMVLCNRA (104–114) | 109 110 | 106 113 | 107 109 110 | |
| TYDNE (125–129) | - | - | 125 126 | |
| KRHDGGCRKE (145–154) | - | 145 146 153 | - | |
| KTYGNKCNFCNAVVES (183–198) | 191 | 183 188 | 184 185 191 194 195 196 197 | |
| TLSHFGKC (203–210) | 203 204 206 207 | 209 | 203 204 207 | |
| Jarvinen [46] | AEVDCSRFPN (25–34) | 32 | 31 | 25 26 27 32 |
| ATDKEGKDVL (35–44) | 32 | 38 41 | 35 36 39 43 | |
| SIEFGTNISK (71–80) | 73 74 | - | 70 72 73 74 76 78 | |
| VEQGASVDKR (137–146) | - | 145 | 137 138 141 143 | |
| Martínez-Botas et al. [41] | DCSRFPNATDKEGKDVL (28–44) | 32 | 31 38 41 | 32 35 36 39 43 |
| YSIEFGTNISKEHD (70–83) | 73 74 | 80 | 70 72 73 74 76 78 81 | |
| FNPVCGTDGVTYDN (115–128) | - | - | 115 118 121 124 125 126 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brumă, M.; Vasilean, I.; Grigore-Gurgu, L.; Banu, I.; Aprodu, I. Advances in Understanding the Antioxidant and Antigenic Properties of Egg-Derived Peptides. Molecules 2024, 29, 1327. https://doi.org/10.3390/molecules29061327
Brumă M, Vasilean I, Grigore-Gurgu L, Banu I, Aprodu I. Advances in Understanding the Antioxidant and Antigenic Properties of Egg-Derived Peptides. Molecules. 2024; 29(6):1327. https://doi.org/10.3390/molecules29061327
Chicago/Turabian StyleBrumă (Călin), Mihaela, Ina Vasilean, Leontina Grigore-Gurgu, Iuliana Banu, and Iuliana Aprodu. 2024. "Advances in Understanding the Antioxidant and Antigenic Properties of Egg-Derived Peptides" Molecules 29, no. 6: 1327. https://doi.org/10.3390/molecules29061327
APA StyleBrumă, M., Vasilean, I., Grigore-Gurgu, L., Banu, I., & Aprodu, I. (2024). Advances in Understanding the Antioxidant and Antigenic Properties of Egg-Derived Peptides. Molecules, 29(6), 1327. https://doi.org/10.3390/molecules29061327