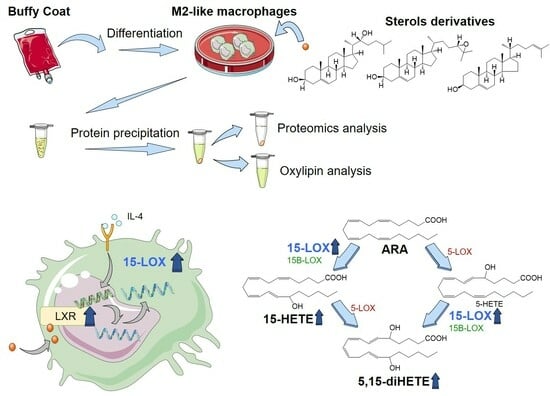
Sterol Derivatives Specifically Increase Anti-Inflammatory Oxylipin Formation in M2-like Macrophages by LXR-Mediated Induction of 15-LOX
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cultivation of Macrophages from Human PBMC
4.3. Analysis of Oxylipins and Protein Levels by LC-MS/MS
4.4. Immunofluorescence Labelling
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qu, X.; Tang, Y.; Hua, S. Immunological Approaches Towards Cancer and Inflammation: A Cross Talk. Front. Immunol. 2018, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, T.; Matsushima, K. Macrophage signaling, apoptosis, lectins and leukocyte trafficking. Trends Immunol. 2001, 22, 593–594. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Gomez, A.; Perretti, M.; Soehnlein, O. Resolution of inflammation: An integrated view. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef]
- Gately, M.K.; Renzetti, L.M.; Magram, J.; Stern, A.S.; Adorini, L.; Gubler, U.; Presky, D.H. The interleukin-12/interleukin-12-receptor system: Role in normal and pathologic immune responses. Annu. Rev. Immunol. 1998, 16, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Rot, A.; von Andrian, U.H. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 2004, 22, 891–928. [Google Scholar] [CrossRef] [PubMed]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef]
- Fleetwood, A.J.; Lawrence, T.; Hamilton, J.A.; Cook, A.D. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: Implications for CSF blockade in inflammation. J. Immunol. 2007, 178, 5245–5252. [Google Scholar] [CrossRef]
- Nathan, C.F.; Murray, H.W.; Wiebe, M.E.; Rubin, B.Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 1983, 158, 670–689. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert. Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef]
- Michlewska, S.; Dransfield, I.; Megson, I.L.; Rossi, A.G. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: Key role for TNF-alpha. FASEB J. 2009, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Roszer, T. Transcriptional control of apoptotic cell clearance by macrophage nuclear receptors. Apoptosis 2017, 22, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.T.; Welch, J.S.; Ricote, M.; Binder, C.J.; Willson, T.M.; Kelly, C.; Witztum, J.L.; Funk, C.D.; Conrad, D.; Glass, C.K. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature 1999, 400, 378–382. [Google Scholar] [CrossRef]
- Roszer, T.; Menendez-Gutierrez, M.P.; Lefterova, M.I.; Alameda, D.; Nunez, V.; Lazar, M.A.; Fischer, T.; Ricote, M. Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator-activated receptor gamma or retinoid X receptor alpha deficiency. J. Immunol. 2011, 186, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Schulman, I.G. Liver X receptors link lipid metabolism and inflammation. FEBS Lett. 2017, 591, 2978–2991. [Google Scholar] [CrossRef]
- Noelia, A.; Bensinger, S.J.; Hong, C.; Beceiro, S.; Bradley, M.N.; Zelcer, N.; Deniz, J.; Ramirez, C.; Diaz, M.; Gallardo, G.; et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 2009, 31, 245–258. [Google Scholar]
- Edwards, P.A.; Kennedy, M.A.; Mak, P.A. LXRs; oxysterol-activated nuclear receptors that regulate genes controlling lipid homeostasis. Vascul. Pharmacol. 2002, 38, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Tontonoz, P. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr. Opin. Genet. Dev. 2008, 18, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Repa, J.J.; Mangelsdorf, D.J. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu. Rev. Cell Dev. Biol. 2000, 16, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, G.Y.; Kim, J.I.; Ham, M.; Won Lee, J.; Kim, J.B. Inhibitory effect of LXR activation on cell proliferation and cell cycle progression through lipogenic activity. J. Lipid Res. 2010, 51, 3425–3433. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, K.R.; Gustafsson, J.A. Putative metabolic effects of the liver X receptor (LXR). Diabetes 2004, 53 (Suppl. S1), S36–S42. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014, 7, a016311. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Kahnt, A.S.; Schebb, N.H.; Steinhilber, D. Formation of lipoxins and resolvins in human leukocytes. Prostaglandins Other Lipid Mediat. 2023, 166, 106726. [Google Scholar] [CrossRef]
- Schebb, N.H.; Kuhn, H.; Kahnt, A.S.; Rund, K.M.; O’Donnell, V.B.; Flamand, N.; Peters-Golden, M.; Jakobsson, P.J.; Weylandt, K.H.; Rohwer, N.; et al. Formation, Signaling and Occurrence of Specialized Pro-Resolving Lipid Mediators-What is the Evidence so far? Front. Pharmacol. 2022, 13, 838782. [Google Scholar] [CrossRef]
- Snodgrass, R.G.; Benatzy, Y.; Schmid, T.; Namgaladze, D.; Mainka, M.; Schebb, N.H.; Lutjohann, D.; Brune, B. Efferocytosis potentiates the expression of arachidonate 15-lipoxygenase (ALOX15) in alternatively activated human macrophages through LXR activation. Cell Death Differ. 2021, 28, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Glass, C.K. Macrophages, oxysterols and atherosclerosis. Circ. J. 2010, 74, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.; Kuhn, H.; Heydeck, D. Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15). Gene 2015, 573, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Banthiya, S.; van Leyen, K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta 2015, 1851, 308–330. [Google Scholar] [CrossRef]
- Rohwer, N.; Chiu, C.Y.; Huang, D.; Smyl, C.; Rothe, M.; Rund, K.M.; Helge Schebb, N.; Kuhn, H.; Weylandt, K.H. Omega-3 fatty acids protect from colitis via an Alox15-derived eicosanoid. FASEB J. 2021, 35, e21491. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef]
- Hartung, N.M.; Mainka, M.; Pfaff, R.; Kuhn, M.; Biernacki, S.; Zinnert, L.; Schebb, N.H. Development of a quantitative proteomics approach for cyclooxygenases and lipoxygenases in parallel to quantitative oxylipin analysis allowing the comprehensive investigation of the arachidonic acid cascade. Anal. Bioanal. Chem. 2023, 415, 913–933. [Google Scholar] [CrossRef]
- Ebert, R.; Cumbana, R.; Lehmann, C.; Kutzner, L.; Toewe, A.; Ferreiros, N.; Parnham, M.J.; Schebb, N.H.; Steinhilber, D.; Kahnt, A.S. Long-term stimulation of toll-like receptor-2 and -4 upregulates 5-LO and 15-LO-2 expression thereby inducing a lipid mediator shift in human monocyte-derived macrophages. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158702. [Google Scholar] [CrossRef]
- Snodgrass, R.G.; Brune, B. Regulation and Functions of 15-Lipoxygenases in Human Macrophages. Front. Pharmacol. 2019, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Kronke, G.; Katzenbeisser, J.; Uderhardt, S.; Zaiss, M.M.; Scholtysek, C.; Schabbauer, G.; Zarbock, A.; Koenders, M.I.; Axmann, R.; Zwerina, J.; et al. 12/15-lipoxygenase counteracts inflammation and tissue damage in arthritis. J. Immunol. 2009, 183, 3383–3389. [Google Scholar] [CrossRef] [PubMed]
- Gronert, K.; Maheshwari, N.; Khan, N.; Hassan, I.R.; Dunn, M.; Laniado Schwartzman, M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J. Biol. Chem. 2005, 280, 15267–15278. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Ishihara, T.; Isobe, Y.; Kato, T.; Kuba, K.; Imai, Y.; Uchino, Y.; Tsubota, K.; Arita, M. Eosinophils promote corneal wound healing via the 12/15-lipoxygenase pathway. FASEB J. 2020, 34, 12492–12501. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Walther, M.; Kuban, R.J. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002, 68–69, 263–290. [Google Scholar]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed]
- Naruhn, S.; Meissner, W.; Adhikary, T.; Kaddatz, K.; Klein, T.; Watzer, B.; Muller-Brusselbach, S.; Muller, R. 15-hydroxyeicosatetraenoic acid is a preferential peroxisome proliferator-activated receptor beta/delta agonist. Mol. Pharmacol. 2010, 77, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Takata, S.; Papayianni, A.; Matsubara, M.; Jimenez, W.; Pronovost, P.H.; Brady, H.R. 15-Hydroxyeicosatetraenoic acid inhibits neutrophil migration across cytokine-activated endothelium. Am. J. Pathol. 1994, 145, 541–549. [Google Scholar] [PubMed]
- Smith, R.J.; Justen, J.M.; Nidy, E.G.; Sam, L.M.; Bleasdale, J.E. Transmembrane signaling in human polymorphonuclear neutrophils: 15(S)-hydroxy-(5Z,8Z,11Z,13E)-eicosatetraenoic acid modulates receptor agonist-triggered cell activation. Proc. Natl. Acad. Sci. USA 1993, 90, 7270–7274. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot. Essent. Fatty Acids 2005, 73, 141–162. [Google Scholar] [CrossRef]
- Ferrante, J.V.; Ferrante, A. Novel role of lipoxygenases in the inflammatory response: Promotion of TNF mRNA decay by 15-hydroperoxyeicosatetraenoic acid in a monocytic cell line. J. Immunol. 2005, 174, 3169–3172. [Google Scholar] [CrossRef]
- Morita, E.; Schroder, J.M.; Christophers, E. Identification of a novel and highly potent eosinophil chemotactic lipid in human eosinophils treated with arachidonic acid. J. Immunol. 1990, 144, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.S.; Gravel, S.; MacLeod, R.J.; Mills, E.; Hashefi, M. Stimulation of human neutrophils by 5-oxo-6,8,11,14-eicosatetraenoic acid by a mechanism independent of the leukotriene B4 receptor. J. Biol. Chem. 1993, 268, 9280–9286. [Google Scholar] [CrossRef] [PubMed]
- Libreros, S.; Shay, A.E.; Nshimiyimana, R.; Fichtner, D.; Martin, M.J.; Wourms, N.; Serhan, C.N. A New E-Series Resolvin: RvE4 Stereochemistry and Function in Efferocytosis of Inflammation-Resolution. Front. Immunol. 2020, 11, 631319. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.C.; Kalyanaraman, C.; Tourdot, B.E.; Conrad, W.S.; Akinkugbe, O.; Freedman, J.C.; Holinstat, M.; Jacobson, M.P.; Holman, T.R. 15-Lipoxygenase-1 biosynthesis of 7S,14S-diHDHA implicates 15-lipoxygenase-2 in biosynthesis of resolvin D5. J. Lipid Res. 2020, 61, 1087–1103. [Google Scholar] [CrossRef]
- Chiang, N.; Fredman, G.; Backhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef] [PubMed]
- De Marino, S.; Carino, A.; Masullo, D.; Finamore, C.; Marchiano, S.; Cipriani, S.; Di Leva, F.S.; Catalanotti, B.; Novellino, E.; Limongelli, V.; et al. Hyodeoxycholic acid derivatives as liver X receptor alpha and G-protein-coupled bile acid receptor agonists. Sci. Rep. 2017, 7, 43290. [Google Scholar] [CrossRef] [PubMed]
- Farnegardh, M.; Bonn, T.; Sun, S.; Ljunggren, J.; Ahola, H.; Wilhelmsson, A.; Gustafsson, J.A.; Carlquist, M. The three-dimensional structure of the liver X receptor beta reveals a flexible ligand-binding pocket that can accommodate fundamentally different ligands. J. Biol. Chem. 2003, 278, 38821–38828. [Google Scholar] [CrossRef] [PubMed]
- Krasowski, M.D.; Ni, A.; Hagey, L.R.; Ekins, S. Evolution of promiscuous nuclear hormone receptors: LXR, FXR, VDR, PXR, and CAR. Mol. Cell Endocrinol. 2011, 334, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Reschly, E.J.; Ai, N.; Welsh, W.J.; Ekins, S.; Hagey, L.R.; Krasowski, M.D. Ligand specificity and evolution of liver X receptors. J. Steroid Biochem. Mol. Biol. 2008, 110, 83–94. [Google Scholar] [CrossRef]
- Heitel, P.; Achenbach, J.; Moser, D.; Proschak, E.; Merk, D. DrugBank screening revealed alitretinoin and bexarotene as liver X receptor modulators. Bioorg. Med. Chem. Lett. 2017, 27, 1193–1198. [Google Scholar] [CrossRef]
- Landis, M.S.; Patel, H.V.; Capone, J.P. Oxysterol activators of liver X receptor and 9-cis-retinoic acid promote sequential steps in the synthesis and secretion of tumor necrosis factor-alpha from human monocytes. J. Biol. Chem. 2002, 277, 4713–4721. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.M.; Kliewer, S.A.; Moore, L.B.; Smith-Oliver, T.A.; Oliver, B.B.; Su, J.L.; Sundseth, S.S.; Winegar, D.A.; Blanchard, D.E.; Spencer, T.A.; et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 1997, 272, 3137–3140. [Google Scholar] [CrossRef] [PubMed]
- Janowski, B.A.; Willy, P.J.; Devi, T.R.; Falck, J.R.; Mangelsdorf, D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 1996, 383, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Peet, D.J.; Janowski, B.A.; Mangelsdorf, D.J. The LXRs: A new class of oxysterol receptors. Curr. Opin. Genet. Dev. 1998, 8, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Janowski, B.A.; Grogan, M.J.; Jones, S.A.; Wisely, G.B.; Kliewer, S.A.; Corey, E.J.; Mangelsdorf, D.J. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc. Natl. Acad. Sci. USA 1999, 96, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Olkkonen, V.M.; Lehto, M. Oxysterols and oxysterol binding proteins: Role in lipid metabolism and atherosclerosis. Ann. Med. 2004, 36, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.H.; Argmann, C.A.; Edwards, J.Y.; Sawyez, C.G.; Morand, O.H.; Hegele, R.A.; Huff, M.W. Enhanced synthesis of the oxysterol 24(S),25-epoxycholesterol in macrophages by inhibitors of 2,3-oxidosqualene:lanosterol cyclase: A novel mechanism for the attenuation of foam cell formation. Circ. Res. 2003, 93, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Mol, M.J.; de Rijke, Y.B.; Demacker, P.N.; Stalenhoef, A.F. Plasma levels of lipid and cholesterol oxidation products and cytokines in diabetes mellitus and cigarette smoking: Effects of vitamin E treatment. Atherosclerosis 1997, 129, 169–176. [Google Scholar] [CrossRef]
- Vejux, A.; Samadi, M.; Lizard, G. Contribution of cholesterol and oxysterols in the physiopathology of cataract: Implication for the development of pharmacological treatments. J. Ophthalmol. 2011, 2011, 471947. [Google Scholar] [CrossRef]
- Wang, D.Q.; Afdhal, N.H. Good cholesterol, bad cholesterol: Role of oxysterols in biliary tract diseases. Gastroenterology 2001, 121, 216–218. [Google Scholar] [CrossRef]
- Javitt, N.B.; Javitt, J.C. The retinal oxysterol pathway: A unifying hypothesis for the cause of age-related macular degeneration. Curr. Opin. Ophthalmol. 2009, 20, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Jeitner, T.M.; Voloshyna, I.; Reiss, A.B. Oxysterol derivatives of cholesterol in neurodegenerative disorders. Curr. Med. Chem. 2011, 18, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Muse, E.D.; Yu, S.; Edillor, C.R.; Tao, J.; Spann, N.J.; Troutman, T.D.; Seidman, J.S.; Henke, A.; Roland, J.T.; Ozeki, K.A.; et al. Cell-specific discrimination of desmosterol and desmosterol mimetics confers selective regulation of LXR and SREBP in macrophages. Proc. Natl. Acad. Sci. USA 2018, 115, E4680–E4689. [Google Scholar] [CrossRef]
- Olkkonen, V.M.; Beaslas, O.; Nissila, E. Oxysterols and their cellular effectors. Biomolecules 2012, 2, 76–103. [Google Scholar] [CrossRef]
- Rigamonti, E.; Chinetti-Gbaguidi, G.; Staels, B. Regulation of macrophage functions by PPAR-alpha, PPAR-gamma, and LXRs in mice and men. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1050–1059. [Google Scholar] [CrossRef]
- Joseph, S.B.; Castrillo, A.; Laffitte, B.A.; Mangelsdorf, D.J.; Tontonoz, P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 2003, 9, 213–219. [Google Scholar] [CrossRef]
- Castrillo, A.; Joseph, S.B.; Marathe, C.; Mangelsdorf, D.J.; Tontonoz, P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J. Biol. Chem. 2003, 278, 10443–10449. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Rund, K.M.; Ostermann, A.I.; Kutzner, L.; Galano, J.M.; Oger, C.; Vigor, C.; Wecklein, S.; Seiwert, N.; Durand, T.; Schebb, N.H. Development of an LC-ESI(-)-MS/MS method for the simultaneous quantification of 35 isoprostanes and isofurans derived from the major n3- and n6-PUFAs. Anal. Chim. Acta 2018, 1037, 63–74. [Google Scholar] [CrossRef]
- Kutzner, L.; Rund, K.M.; Ostermann, A.I.; Hartung, N.M.; Galano, J.M.; Balas, L.; Durand, T.; Balzer, M.S.; David, S.; Schebb, N.H. Development of an Optimized LC-MS Method for the Detection of Specialized Pro-Resolving Mediators in Biological Samples. Front. Pharmacol. 2019, 10, 169. [Google Scholar] [CrossRef]
- Koch, E.; Mainka, M.; Dalle, C.; Ostermann, A.I.; Rund, K.M.; Kutzner, L.; Froehlich, L.F.; Bertrand-Michel, J.; Gladine, C.; Schebb, N.H. Stability of oxylipins during plasma generation and long-term storage. Talanta 2020, 217, 121074. [Google Scholar] [CrossRef] [PubMed]
- Hartung, N.M.; Ostermann, A.I.; Immenschuh, S.; Schebb, N.H. Combined Targeted Proteomics and Oxylipin Metabolomics for Monitoring of the COX-2 Pathway. Proteomics 2021, 21, e1900058. [Google Scholar] [CrossRef] [PubMed]
- Bornhorst, J.; Wehe, C.A.; Huwel, S.; Karst, U.; Galla, H.J.; Schwerdtle, T. Impact of manganese on and transfer across blood-brain and blood-cerebrospinal fluid barrier in vitro. J. Biol. Chem. 2012, 287, 17140–17151. [Google Scholar] [CrossRef]
- Mallick, P.; Schirle, M.; Chen, S.S.; Flory, M.R.; Lee, H.; Martin, D.; Ranish, J.; Raught, B.; Schmitt, R.; Werner, T.; et al. Computational prediction of proteotypic peptides for quantitative proteomics. Nat. Biotechnol. 2007, 25, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [PubMed]
- Gaudet, P.; Michel, P.A.; Zahn-Zabal, M.; Britan, A.; Cusin, I.; Domagalski, M.; Duek, P.D.; Gateau, A.; Gleizes, A.; Hinard, V.; et al. The neXtProt knowledgebase on human proteins: 2017 update. Nucleic Acids Res. 2017, 45, D177–D182. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Fannes, T.; Vandermarliere, E.; Schietgat, L.; Degroeve, S.; Martens, L.; Ramon, J. Predicting tryptic cleavage from proteomics data using decision tree ensembles. J. Proteome Res. 2013, 12, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- UniProt, C. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. [Google Scholar] [CrossRef]
- Hoofnagle, A.N.; Whiteaker, J.R.; Carr, S.A.; Kuhn, E.; Liu, T.; Massoni, S.A.; Thomas, S.N.; Townsend, R.R.; Zimmerman, L.J.; Boja, E.; et al. Recommendations for the Generation, Quantification, Storage, and Handling of Peptides Used for Mass Spectrometry-Based Assays. Clin. Chem. 2016, 62, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Lange, V.; Picotti, P.; Domon, B.; Aebersold, R. Selected reaction monitoring for quantitative proteomics: A tutorial. Mol. Syst. Biol. 2008, 4, 222. [Google Scholar] [CrossRef] [PubMed]
- Gallien, S.; Duriez, E.; Domon, B. Selected reaction monitoring applied to proteomics. J. Mass. Spectrom. 2011, 46, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Krokhin, O.V.; Spicer, V. Peptide retention standards and hydrophobicity indexes in reversed-phase high-performance liquid chromatography of peptides. Anal. Chem. 2009, 81, 9522–9530. [Google Scholar] [CrossRef]
- Kusebauch, U.; Campbell, D.S.; Deutsch, E.W.; Chu, C.S.; Spicer, D.A.; Brusniak, M.Y.; Slagel, J.; Sun, Z.; Stevens, J.; Grimes, B.; et al. Human SRMAtlas: A Resource of Targeted Assays to Quantify the Complete Human Proteome. Cell 2016, 166, 766–778. [Google Scholar] [CrossRef]





| Time-Dependent Incubations [pmol/mg Protein] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ctrl | 3 h | 6 h | 18 h | 24 h | 30 h | 48 h | ||
| Oxylipin concentration | 12-HETE | 15.5 ± 2.2 | 22.7 ± 2.2 | 26.4 ± 3.3 | 27.1 ± 3.4 | 32.5 ± 3.4 | 33.5 ± 8.9 | 29.3 ± 2.9 |
| 15-HETE | 184 ± 29 | 256 ± 40 | 277 ± 34 | 280 ± 25 | 327 ± 16 | 476 ± 213 | 364 ± 100 | |
| 5,15-diHETE | 9.18 ± 1.1 | 25.6 ± 4.9 | 28.3 ± 2.8 | 33.7 ± 1.3 | 45.9 ± 8.2 | 59.6 ± 20 | 57.3 ± 6.4 | |
| 5,12-diHETE | 0.47 ± 0.1 | 1.00 ± 0.4 | 1.23 ± 0.2 | 1.76 ± 0.4 | 2.17 ± 0.2 | 1.11 ± 0.4 | 0.64 ± 0.1 | |
| 5,15-diHEPE | 3.30 ± 0.4 | 7.73 ± 0.4 | 9.60 ± 1.4 | 12.9 ± 1.9 | 14.0 ± 2.5 | 10.4 ± 0.9 | 10.2 ± 3.2 | |
| 8,15-diHETE | 0.28 ± 0.1 | 0.55 ± 0.1 | 0.99 ± 0.3 | 0.98 ± 0.2 | 0.87 ± 0.2 | 0.83 ± 0.3 | 0.64 ± 0.5 | |
| 7,17-diHDHA | 10.7 ± 1.0 | 21.1 ± 3.7 | 25.5 ± 0.8 | 33.1 ± 1.3 | 40.4 ± 1.7 | 56.9 ± 17 | 39.5 ± 5.3 | |
| 12-HEPE | 1.60 ± 0.3 | 1.93 ± 0.2 | 2.29 ± 0.3 | 2.32 ± 0.1 | 2.11 ± 0.5 | 1.20 ± 0.9 | 1.23 ± 0.7 | |
| 15-HEPE | 18.9 ± 2.9 | 22.1 ± 2.7 | 26.1 ± 3.1 | 28.83 ± 1.8 | 22.1 ± 4.1 | 11.7 ± 8.9 | 12.1 ± 8.5 | |
| 14-HDHA | 16.2 ± 4.0 | 18.3 ± 3.3 | 22.3 ± 4.1 | 24.7 ± 2.0 | 25.0 ± 7.5 | 14.2 ± 12 | 14.4 ± 14 | |
| 7-HDHA | 3.65 ± 1.6 | 2.46 ± 0.8 | 2.89 ± 1.0 | 2.86 ± 0.4 | 2.98 ± 1.1 | 2.13 ± 1.8 | 2.18 ± 1.5 | |
| 17-HDHA | 45.4 ± 14 | 40.3 ± 8.5 | 51.4 ± 13 | 52.2 ± 5.9 | 50.7 ± 15 | 33.3 ± 29 | 34.0 ± 39 | |
| 15-LOX level | 2.95 ± 0.6 | 4.40 ± 0.9 | 4.81 ± 0.6 | 5.66 ± 1.1 | 5.95 ± 0.8 | 2.04 ± 1.0 | 1.76 ± 0.6 | |
| Dose-Dependent Incubations [pmol/mg Protein] | ||||||||
| Ctrl | 0.3 µM | 1 µM | 3 µM | 10 µM | ||||
| Oxylipin concentration | 12-HETE | 2.78 ± 1.0 | 5.55 ± 2.1 | 6.86 ± 2.9 | 7.53 ± 2.7 | 10.1 ± 4.0 | ||
| 15-HETE | 39.9 ± 20 | 66.2 ± 33 | 86.3 ± 31 | 85.3 ± 31 | 126 ± 53 | |||
| 5,15-diHETE | 1.02 ± 0.7 | 1.93 ± 1.6 | 2.73 ± 1.3 | 2.10 ± 1.0 | 4.66 ± 3.1 | |||
| 5,12-diHETE | 0.22 ± 0.1 | 0.20 ± 0.1 | 0.30 ± 0.1 | 0.23 ± 0.1 | 0.34 ± 0.2 | |||
| 5,15-diHEPE | 0.37 ± 0.1 | 0.80 ± 0.2 | 1.11 ± 0.3 | 1.29 ± 0.5 | 1.68 ± 0.8 | |||
| 8,15-diHETE | 0.14 ± 0.1 | 0.13 ± 0.1 | 0.20 ± 0.02 | 0.06 ± 0.02 | 0.20 ± 0.05 | |||
| 7,17-diHDHA | 0.71 ± 0.4 | 1.79 ± 1.2 | 1.83 ± 0.9 | 2.34 ± 1.8 | 3.94 ± 2.6 | |||
| 12-HEPE | 0.32 ± 0.14 | 0.80 ± 0.3 | 0.83 ± 0.3 | 0.80 ± 0.2 | 1.04 ± 0.3 | |||
| 15-HEPE | 5.20 ± 2.7 | 9.29 ± 4.5 | 10.2 ± 3.8 | 9.00 ± 2.7 | 12.4 ± 4.4 | |||
| 14-HDHA | 1.79 ± 0.8 | 4.51 ± 2.1 | 4.83 ± 2.1 | 5.43 ± 1.9 | 6.07 ± 2.2 | |||
| 7-HDHA | 0.88 ± 0.5 | 1.63 ± 1.0 | 1.97 ± 0.9 | 1.60 ± 0.5 | 1.50 ± 0.6 | |||
| 17-HDHA | 6.32 ± 3.4 | 14.4 ± 8.4 | 15.5 ± 6.2 | 15.4 ± 5.3 | 18.4 ± 7.1 | |||
| 15-LOX level | 0.77 ± 0.1 | 1.00 ± 0.2 | 1.29 ± 0.2 | 1.22 ± 0.1 | 1.13 ± 0.1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohno, R.; Mainka, M.; Kirchhoff, R.; Hartung, N.M.; Schebb, N.H. Sterol Derivatives Specifically Increase Anti-Inflammatory Oxylipin Formation in M2-like Macrophages by LXR-Mediated Induction of 15-LOX. Molecules 2024, 29, 1745. https://doi.org/10.3390/molecules29081745
Ohno R, Mainka M, Kirchhoff R, Hartung NM, Schebb NH. Sterol Derivatives Specifically Increase Anti-Inflammatory Oxylipin Formation in M2-like Macrophages by LXR-Mediated Induction of 15-LOX. Molecules. 2024; 29(8):1745. https://doi.org/10.3390/molecules29081745
Chicago/Turabian StyleOhno, Reiichi, Malwina Mainka, Rebecca Kirchhoff, Nicole M. Hartung, and Nils Helge Schebb. 2024. "Sterol Derivatives Specifically Increase Anti-Inflammatory Oxylipin Formation in M2-like Macrophages by LXR-Mediated Induction of 15-LOX" Molecules 29, no. 8: 1745. https://doi.org/10.3390/molecules29081745
APA StyleOhno, R., Mainka, M., Kirchhoff, R., Hartung, N. M., & Schebb, N. H. (2024). Sterol Derivatives Specifically Increase Anti-Inflammatory Oxylipin Formation in M2-like Macrophages by LXR-Mediated Induction of 15-LOX. Molecules, 29(8), 1745. https://doi.org/10.3390/molecules29081745