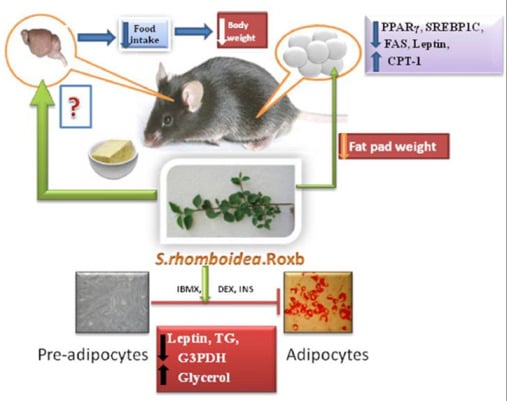
Sida rhomboidea. Roxb Leaf Extract Down-Regulates Expression of PPARγ2 and Leptin Genes in High Fat Diet Fed C57BL/6J Mice and Retards in Vitro 3T3L1 Pre-Adipocyte Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Extract
2.3. Experimental Animals
2.3.1. High Fat Diet Induced Obesity in C57BL/6J Mice
2.3.2. Plasma Lipids and Leptin Assay
2.3.3. Analysis of Gene Expression by Quantitative RT-PCR (qPCR)
2.3.4. Microscopic and Morphometric Examination of Epididymal Fat Pad
2.4. Maintenance of 3T3L1 Cells
2.4.1. In Vitro Cytotoxicity Assay
2.4.2. In Vitro Adipocyte Differentiation Protocol
2.4.3. Qualitative and Quantitative Analysis of Adipocyte Differentiation
2.4.4. Leptin Release and Triglyceride Accumulation Assays
2.4.5. Glycerol Release Assay
2.4.6. G3PDH Activity Assay
2.5. Statistical Analysis
3. Results
3.1. Body Weight Gain, Food Intake and Feed Efficiency
3.2. Plasma Lipids and Leptin
3.3. Visceral Adiposity
3.4. Microscopic and Morphometric Evaluation of Epididymal Fat Pad
3.5. Quantitative RT PCR Analysis
3.6. Cytotoxicity Assay
3.7. Qualitative and Quantitative Analysis of Adipocyte Differentiation
3.8. Trigyceride Accumulation and Leptin Release in Differentiated Adipocytes
3.9. Glycerol Release and G3PDH Activity Assay
4. Discussion
5. Conclusion
Acknowledgement
References
- World Health Organization. World Health Organization Fact Sheet for Worldwide Prevalence of Obesity. 2010. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/index.html accessed on 12 January 2011.
- Haffner, SM. Abdominal obesity, insulin resistance, and cardiovascular risk in pre-diabetes and type 2 diabetes. Eur. Heart J 2006, 8, 20–25. [Google Scholar]
- Schuster, DP. Obesity and the development of type 2 diabetes: The effects of fatty tissue inflammation. Diabetes Metab. Syndr. Obes 2010, 3, 253–262. [Google Scholar]
- Barsh, GS; Farooqi, IS; O’Rahilly, S. Genetics of body-weight regulation. Nature 2000, 404, 644–651. [Google Scholar]
- Bråkenhielm, E; Cao, R; Gao, B; Angelin, B; Cannon, B; Parini, P; Cao, Y. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ. Res 2004, 94, 1579–1588. [Google Scholar]
- Yun, JW. Possible anti-obesity therapeutics from nature–A review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar]
- Kang, S; Kim, MH; Shin, HS; Kim, HM; Hong, YS; Park, JG; Ko, HC; Lee, NH; Chung, WS; Kim, SJ. A water-soluble extract of Petalonia binghamiae inhibits the expression of adipogenic regulators in 3T3-L1 pre-adipocytes and reduces adiposity and weight gain in rats fed a high-fat diet. J. Nutr. Biochem 2010, 21, 1251–1257. [Google Scholar]
- Ramachandra Rao, R; Sudarshan, SK. Encyclopaedia of Indian Medicine, 4th ed; Dr. V. Parameshvara Charitable Trust: Bangalore, India, 2005; pp. 34–35. [Google Scholar]
- Prakash, A; Verma, RK; Ghosal, S. Alkaloidal constituents of Sida acuta, S. rhombifolia and S. spinosa. Plant. Med 1981, 43, 384–388. [Google Scholar]
- Goyal, MM; Rani, KK. Effect of natural products isolated from three species of Sida on some gram-positive and gran-negetive bacteria. J. Ind. Chem. Soc 1988, 65, 74–76. [Google Scholar]
- Goyal, MM; Rani, KK. Neutral constituents of the aerial parts of Sida rhombifolia var. rhomboidea. Fitoterapia 1989, 60, 163–164. [Google Scholar]
- Thounaojam, MC; Jadeja, RN; Ansarullah; Devkar, RV; Ramachandran, AV. Potential of Sida rhomboidea. Roxb leaf extract in controlling hypertriglyceridemia in experimental models. Pharmacogn. Res 2009, 1, 208–212. [Google Scholar]
- Thounaojam, M; Jadeja, R; Ansarullah; Devkar, R; Ramachandran, AV. Dysregulation of lipid and cholesterol metabolism in high fat diet fed hyperlipidemic rats: Protective effect of Sida rhomboidea. Roxb leaf extract. J. Health Sci 2009, 55, 413–420. [Google Scholar]
- Thounaojam, MC; Jadeja, RN; Ansarullah; Devkar, RV; Ramachandran, AV. Prevention of high fat diet induced insulin resistance in C57BL/6J mice by Sida rhomboidea. Roxb extract. J. Health Sci 2010, 56, 92–98. [Google Scholar]
- Thounaojam, MC; Jadeja, RN; Ansarullah; Karn, SS; Shah, JD; Patel, DK; Salunke, SP; Padate, GS; Devkar, RV; Rmachandran, AV. Cardioprotective effect of Sida rhomboidea. Roxb extract against isoproterenol induced myocardial necrosis in rats. Exp. Toxicol. Pathol 2011, 63, 351–356. [Google Scholar]
- Thounaojam, MC; Jadeja, RN; Dandekar, DS; Devkar, RV; Ramachandran, AV. Sida rhomboidea. Roxb extract alleviates pathophysiological changes in experimental in vivo and in vitro models of high fat diet/fatty acid induced non-alcoholic steatohepatitis. Exp Toxicol Pathol 2010. [Google Scholar] [CrossRef]
- Thounaojam, MC; Jadeja, RN; Devkar, RV; Ramachandran, AV. In Vitro evidence for the protective role of Sida rhomboidea. Roxb extract against LDL oxidation and oxidized LDL-induced apoptosis in human monocyte-derived macrophages. Cardiovasc. Toxicol 2011, 11, 168–179. [Google Scholar]
- Thounaojam, MC; Jadeja, RN; Patel, DK; Devkar, RV; Ramachandran, AV. Acute and sub chronic oral toxicity of Sida rhomboidea.Roxb leaf extract. J Complement Integr Med 2010, 7, 1:1–1:6. [Google Scholar]
- Kumar, TP; Antony, S; Gireesh, G; George, N; Paulose, CS. Curcumin modulates dopaminergic receptor, CREB and phospholipase c gene expression in the cerebral cortex and cerebellum of streptozotocin induced diabetic rats. J. Biomed. Sci 2010, 43, 1–11. [Google Scholar]
- Jadeja, RN; Thounaojam, MC; Ramani, UV; Devkar, RV; Ramachandran, AV. Anti-obesity potential of Clerodendron glandulosum. Coleb leaf aqueous extract. J. Ethnopharmacol 2011, 135, 338–343. [Google Scholar]
- Sturgeon, RJ; Deamer, RL; Harbison, HA. Improved spectrophotometric determination of glycerol and its comparison with an enzymatic method. J. Pharm. Sci 1979, 68, 1064–1066. [Google Scholar]
- Wise, LS; Green, H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J. Biol. Chem 1979, 254, 273–275. [Google Scholar]
- Lowry, OH; Rosebrough, NJ; Farr, AL; Randall, RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem 1951, 193, 265–275. [Google Scholar]
- West, KM; Kalbfleisch, JM. Influence of nutritional factors on prevalence of diabetes. Diabetes 1971, 20, 99–108. [Google Scholar]
- Rebuffe-Scrive, M; Surwit, R; Feinglos, M; Kuhn, C; Rodin, J. Regional fat distribution and metabolism in a new mouse model (C57BL/6J) of non-insulin-dependent diabetes mellitus. Metabolism 1993, 42, 1405–1409. [Google Scholar]
- Surwit, RS; Feinglos, MN; Rodin, J; Sutherland, A; Petro, AE; Opara, EC; Kuhn, CM; Rebuffé-Scrive, M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism 1995, 44, 645–651. [Google Scholar]
- Kim, MJ; Kim, HK. Perilla leaf extract ameliorates obesity and dyslipidemia induced by high-fat diet. Phytother. Res 2009, 23, 1685–1690. [Google Scholar]
- Kim, NH; Choi, SK; Kim, SJ; Moon, PD; Lim, HS; Choi, IY; Na, HJ; An, HJ; Myung, NY; Jeong, HJ; Um, JY; Hong, SH; Kim, HM. Green tea seed oil reduces weight gain in C57BL/6J mice and influences adipocyte differentiation by suppressing peroxisome proliferator-activated receptor-gamma. Pflugers Arch 2008, 457, 293–302. [Google Scholar]
- Singh, AP. Bala (Sida cordifolia L.)—Is it safe herbal drug? Ethnobot. Leafl 2006, 10, 336–341. [Google Scholar]
- Khatoon, S; Srivastava, M; Rawat, AKS; Mehrotra, S. HPTLC method for chemical standardization of Sida species and estimation of the alkaloid ephedrine. J. Planar Chromatogr 2005, 18, 364–367. [Google Scholar]
- Astrup, A; Breum, L; Toubro, S. Pharmacological and clinical studies of ephedrine and other thermogenic agonists. Obes. Res 1995, 3, 537S–540S. [Google Scholar]
- Carek, PJ; Dickerson, LM. Current concepts in the pharmacological management of obesity. Drugs 1999, 57, 883–904. [Google Scholar]
- Cristin, M. Ephedra falls under FDA jurisdiction—natural stimulant. 1997. Available online: http://findarticles.com/p/articles/mi_m0820/is_n241/ai_19733239/ accessed on 5 January 2011.
- Marques, BG; Hausman, DB; Martin, RJ. Association of fat cell size and paracrine growth factors in development of hyperplastic obesity. Am. J. Physiol 1998, 275, 1898–1908. [Google Scholar]
- Garaulet, M; Hernandez-Morante, JJ; Lujan, J; Tebar, FJ; Zamora, S. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int. J. Obes 2006, 30, 899–905. [Google Scholar]
- Maffei, M; Halaas, J; Ravussin, E; Pratley, RE; Lee, GH; Zhang, Y; Fei, H; Kim, S; Lallone, R; Ranganathan, S. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med 1995, 1, 1155–1161. [Google Scholar]
- Staiger, H; Tschritter, O; Machann, J; Thamer, C; Fritsche, A; Maerker, E; Schick, F; Haring, HU; Stumvoll, M. Relationship of serum adiponectin and leptin concentrations with body fat distribution in humans. Obes. Res 2003, 11, 368–372. [Google Scholar]
- Nerurkar, PV; Lee, YK; Nerurkar, VR. Momordica charantia (bitter melon) inhibits primary human adipocyte differentiation by modulating adipogenic genes. BMC Complement Altern Med 2010, 10, 34:1–34:10. [Google Scholar]
- Rousseau, V; Becker, DJ; Ongemba, LN; Rahier, J; Henquin, JC; Brichard, SM. Developmental and nutritional changes of ob and PPAR gamma 2 gene expression in rat white adipose tissue. Biochem. J 1997, 321, 451–456. [Google Scholar]
- Christensen, KB; Minet, A; Svenstrup, H; Grevsen, K; Zhang, H; Schrader, E; Rimbach, G; Wein, S; Wolffram, S; Kristiansen, K; Christensen, LP. Identification of plant extracts with potential anti-diabetic properties: effect on human peroxisome proliferator activated receptor (PPAR), adipocyte differentiation and insulin-stimulated glucose uptake. Phytother. Res 2009, 23, 1316–1325. [Google Scholar]
- Freise, C; Erben, U; Neuman, U; Kim, K; Zeitz, M; Somasundaram, R; Ruehl, M. An active extract of Lindera obtusiloba inhibits adipogenesis via sustained Wnt signaling and exerts anti-inflammatory effects in the 3T3-L1 pre-adipocytes. J. Nutr. Biochem 2010, 21, 1170–1177. [Google Scholar]
- Brown, MS; Goldstein, JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997, 89, 331–340. [Google Scholar]
- Hsuy, SC; Huang, CJ. Reduced fat mass in rats fed a high oleic acid–rich safflower oil diet is associated with changes in expression of hepatic PPARα and adipose SREBP-1c–regulated genes. J. Nutr 2006, 136, 1779–1785. [Google Scholar]
- Kersten, S. Mechanism of nutritional and hormonal regulation of lipogenesis. EMBO Rep 2001, 21, 282–286. [Google Scholar]
- Kim, JB; Wright, HM; Wright, M; Spiegelman, BM. ADD1/SREBP1 activates PPAR gamma through the production of endogenous ligand. Proc. Natl. Acad. Sci. USA 1998, 95, 4333–4337. [Google Scholar]
- Farmer, SR. Regulation of PPARγ activity during adipogenesis. Int. J. Obes 2005, 29, 13–16. [Google Scholar]
- Schachtrup, CET; Bleck, B; Sandqvist, A; Spener, F. Functional analysis of peroxisome-proliferator-responsive element motifs in genes of fatty acid-binding proteins. Biochem. J 2004, 382, 239–245. [Google Scholar]
- Seo, JB; Choe, SS; Jeong, HW; Park, SW; Shin, HJ; Choi, SM; Park, JY; Choi, EW; Kim, JB; Seen, DS; et al. Anti-obesity effects of Lysimachia foenum-graecum characterized by decreased adipogenesis and regulated lipid metabolism. Exp. Mol. Med 2011, 43, 205–215. [Google Scholar]
- Hong, JH; Hwang, EY; Kim, HJ; Jeong, YJ; Lee, IS. Artemisia capillaris inhibits lipid accumulation in 3T3-L1 adipocytes and obesity in C57BL/6J mice fed a high fat diet. J. Med. Food 2009, 12, 736–745. [Google Scholar]
- Kubota, H; Kojima-Yuasa, A; Morii, R; Huang, X; Norikura, T; Rho, SN; Matsui-Yuasa, I. Anti-obesity effect of Blumea balsamifera extract in 3T3-L1 pre-adipocytes and adipocytes. Am. J. Chin. Med 2009, 37, 843–854. [Google Scholar]






| Ingredients | Low Fat Diet (g/kg) | High Fat Diet (g/kg) |
|---|---|---|
| Casein | 200 | 200 |
| l-Cystine | 3 | 3 |
| Corn Starch | 315 | 0.0 |
| Maltodextrin | 35 | 125 |
| Sucrose | 100 | 68.8 |
| Cellulose | 50 | 50 |
| Soybean Oil | 25 | 25 |
| Lard | 20 | 245 |
| Mineral Mix 1 | 10 | 10 |
| Di Calcium Phosphate | 13 | 13 |
| Calcium Carbonate | 5.5 | 5.5 |
| Potassium Citrate | 16.5 | 16.5 |
| Vitamin Mix 2 | 10 | 10 |
| Choline chloride | 2 | 2 |
| Regular chow | 195 | 216.25 |
| SRLE | 00 | 10 |
| Energy Content | 16.25 kJ/g | 25.72 kJ/g |
| Protein, % of energy | 20 | 20 |
| Carbohydrate, % of energy | 64 | 35 |
| Fat, % of energy | 6 | 45 |
| LEAN | OB | OB+SRLE | |
|---|---|---|---|
| Plasma | |||
| Triglycerides (mmol/L) | 0.53 ± 0.02a | 2.16 ± 0.05b | 0.61 ± 0.04a |
| Free fatty acids (mmol/L) | 1.80 ± 0.07a | 4.68 ± 0.09b | 2.33 ± 0.08a |
| Leptin (ng/L) | 12.00 ± 1.98a | 46.24 ± 2.13b | 25.01 ± 1.89c |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thounaojam, M.C.; Jadeja, R.N.; Ramani, U.V.; Devkar, R.V.; Ramachandran, A.V. Sida rhomboidea. Roxb Leaf Extract Down-Regulates Expression of PPARγ2 and Leptin Genes in High Fat Diet Fed C57BL/6J Mice and Retards in Vitro 3T3L1 Pre-Adipocyte Differentiation. Int. J. Mol. Sci. 2011, 12, 4661-4677. https://doi.org/10.3390/ijms12074661
Thounaojam MC, Jadeja RN, Ramani UV, Devkar RV, Ramachandran AV. Sida rhomboidea. Roxb Leaf Extract Down-Regulates Expression of PPARγ2 and Leptin Genes in High Fat Diet Fed C57BL/6J Mice and Retards in Vitro 3T3L1 Pre-Adipocyte Differentiation. International Journal of Molecular Sciences. 2011; 12(7):4661-4677. https://doi.org/10.3390/ijms12074661
Chicago/Turabian StyleThounaojam, Menaka C., Ravirajsinh N. Jadeja, Umed V. Ramani, Ranjitsinh V. Devkar, and A. V. Ramachandran. 2011. "Sida rhomboidea. Roxb Leaf Extract Down-Regulates Expression of PPARγ2 and Leptin Genes in High Fat Diet Fed C57BL/6J Mice and Retards in Vitro 3T3L1 Pre-Adipocyte Differentiation" International Journal of Molecular Sciences 12, no. 7: 4661-4677. https://doi.org/10.3390/ijms12074661
APA StyleThounaojam, M. C., Jadeja, R. N., Ramani, U. V., Devkar, R. V., & Ramachandran, A. V. (2011). Sida rhomboidea. Roxb Leaf Extract Down-Regulates Expression of PPARγ2 and Leptin Genes in High Fat Diet Fed C57BL/6J Mice and Retards in Vitro 3T3L1 Pre-Adipocyte Differentiation. International Journal of Molecular Sciences, 12(7), 4661-4677. https://doi.org/10.3390/ijms12074661