Fractionation of Whey Protein Isolate with Supercritical Carbon Dioxide—Process Modeling and Cost Estimation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
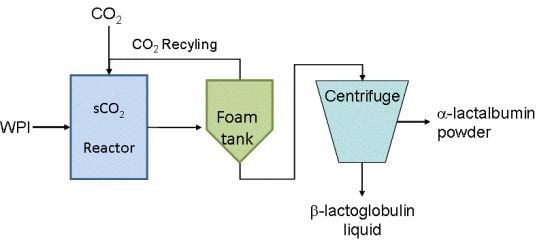
2.2. Pilot-Scale Process Setup and Experimental Protocol
2.3. pH Determination
2.4. Yield and Compositions of the Fractions
2.5. Commercial-Scale Process Design and Modeling
2.6. Centrifugation
2.7. Foam Stability
3. Results and Discussion
3.1. Pilot-Scale Fractionation Results
3.2. Design and Modeling of a Semi-Continuous, Commercial-Scale Process
3.2.1. Design of the Industrial Centrifuge
3.2.2. Design of the De-Foaming Tank
3.2.3. Process Simulation and Economic Analysis vs. Operating Conditions
3.2.4. Effects of Operating Conditions on Production Costs
4. Conclusions
Acknowledgments
References
- Bonnaillie, L.M.; Tomasula, P.M. Whey protein fractionation. In Whey Processing, Functionality and Health Benefits; Onwulata, C.I., Huth, P.J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2008. [Google Scholar]
- Morr, C.V. Whey proteins: Manufacture. In Developments in Dairy Chemistry—4: Functional Milk Proteins; Fox, P.F., Ed.; Elsevier Applied Science: New York, NY, USA, 1989; pp. 245–284. [Google Scholar]
- Pearce, R.J. Fractionation of whey proteins. Aust. J. Dairy Technol 1987, 212, 150. [Google Scholar]
- Outinen, M.; Tossavainen, O.; Tupasela, T.; Koskela, P.; Koskinen, H.; Rantamäki, P.; Syväoja, E.-L.; Antila, P.; Kankare, V. Fractionation of proteins from whey with different pilot scale processes. LWT Food Sci. Technol 1996, 29, 411–417. [Google Scholar]
- Bramaud, C.; Aimar, P.; Daufin, G. Whey protein fractionation: isoelectric precipitation of α-lactalbumin under gentle heat treatment. Biotechnol. Bioeng 1997, 56, 391–397. [Google Scholar]
- Law, A.J.; Leaver, J. Effect of pH on the thermal denaturation of whey proteins in milk. J. Agric. Food Chem 2000, 48, 672–679. [Google Scholar]
- Tolkach, A.; Kulozik, U. Effect of pH and temperature on the reaction kinetic parameters of the thermal denaturation of β-lactoglobulin. Milchwissenschaft-Milk Sci. Int 2005, 60, 248–252. [Google Scholar]
- Bruck, W.M.; Redgrave, M.; Tuohy, K.M.; Lonnerdal, B.; Graverholt, G.; Hernell, O.; Gibson, G.R. Effects of bovine alpha-lactalbumin and casein glycomacropeptide-enriched infant formulae on faecal microbiota in healthy term infants. J. Pediatr. Gastr. Nutr 2006, 43, 673–679. [Google Scholar]
- Sandstrom, O.; Lonnerdal, B.; Graverholt, G.; Hernell, O. Effects of alpha-lactalbumin-enriched formula containing different concentrations of glycomacropeptide on infant nutrition. Am. J. Clin. Nutr 2008, 87, 921–928. [Google Scholar]
- Heine, W.; Radke, M.; Wietzke, K.D.; Polars, E.; Kundt, G. α-Lactalbumin-enriched low-protein infant formulas: A comparison to breast milk feeding. Acta Pediatr 1996, 85, 1024–1028. [Google Scholar]
- Heine, W.E.; Klein, P.D.; Miyashita, C. Method for Isolating Alpha-Lactalbumin from Whey. Patent No. WO 92/03468 1992. [Google Scholar]
- Etzel, M.R. Manufacture and use of dairy protein fractions. J. Nutr 2004, 134, 996S–1002S. [Google Scholar]
- Farrell, H.M., Jr; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the proteins of cows’ milk—Sixth revision. J. Dairy Sci 2004, 87, 1641–1674. [Google Scholar]
- Bonnaillie, L.M.; Tomasula, P.M. Kinetics, aggregation behavior and optimization of the fractionation of whey protein isolate with hydrochloric acid. Food Bioprod. Process.
- Bramaud, C.; Aimar, P.; Daufin, G. Optimisation of a whey protein fractionation process based on the selective precipitation of α-lactalbumin. Lait 1997, 77, 411–423. [Google Scholar]
- Nakamura, K.; Hoshino, T.; Ariyama, H. Adsorption of carbon dioxide on proteins in the supercritical region. Agric. Biol. Chem 1991, 55, 2341–2347. [Google Scholar]
- Chaitanya, V.S.; Senapati, S. Self-assembled reverse micelles in supercritical CO2 entrap protein in native state. J. Am. Chem. Soc 2008, 130, 1866–1870. [Google Scholar]
- Winters, M.A.; Knutson, B.L.; Debenedetti, P.G.; Sparks, H.G.; Przybycien, T.M.; Stevenson, C.L.; Prestrelski, S.J. Precipitation of proteins in supercritical carbon dioxide. J. Pharm. Sci 1996, 85, 586–594. [Google Scholar]
- Maheshwari, P.; Ooi, E.T.; Nikolov, Z.L. Off-flavor removal from soy-protein isolate by using liquid and supercritical carbon-dioxide. J. Am. Oil Chem. Soc 1995, 72, 1107–1115. [Google Scholar]
- Thiering, R.; Hofland, G.; Foster, N.; Witkamp, G.J.; van de Wielen, L. Fractionation of soybean proteins with pressurized carbon dioxide as a volatile electrolyte. Biotechnol. Bioeng 2001, 73, 1–11. [Google Scholar]
- Thiering, R.; Hofland, G.; Foster, N.; Witkamp, G.J.; van de Wielen, L. Carbon dioxide induced soybean protein precipitation: Protein fractionation, particle aggregation, and continuous operation. Biotechnol. Prog 2001, 17, 513–521. [Google Scholar]
- Hofland, G.W.; de Rijke, A.; Thiering, R.; van der Wielen, L.A.M.; Witkamp, G.J. Isoelectric precipitation of soybean protein using carbon dioxide as a volatile acid. J. Chromatogr. B 2000, 743, 357–368. [Google Scholar]
- Khorshid, N.; Hossain, M.M.; Farid, M.M. Precipitation of food protein using high pressure carbon dioxide. J. Food Eng 2007, 79, 1214–1220. [Google Scholar]
- Park, J.Y.; Back, S.S.; Chun, B.S. Protein properties of mackerel viscera extracted by supercritical carbon dioxide. J. Environ. Biol 2008, 29, 443–448. [Google Scholar]
- Kang, K.Y.; Ahn, D.H.; Jung, S.M.; Kim, D.H.; Chun, B.S. Separation of protein and fatty acids from tuna viscera using supercritical carbon dioxide. Biotechnol. Bioproc. Eng 2005, 10, 315–321. [Google Scholar]
- Zhou, L.Y.; Zhang, Y.; Leng, X.J.; Liao, X.J.; Hu, X.S. Acceleration of precipitation formation in peach juice induced by high-pressure carbon dioxide. J. Agric. Food Chem 2010, 58, 9605–9610. [Google Scholar]
- Hirata, G.A.M.; Bernardo, A.; Miranda, E.A. Crystallization of porcine insulin with carbon dioxide as acidifying agent. Powder Technol 2010, 197, 54–57. [Google Scholar]
- Sarkari, M.; Darrat, I.; Knutson, B.L. CO2 and fluorinated solvent-based technologies for protein microparticle precipitation from aqueous solutions. Biotechnol. Prog 2003, 19, 448–454. [Google Scholar]
- Olano, A.; Calvo, M.M.; Troyano, E.; Amigo, L. Changes in the fractions of carbohydrates and whey proteins during heat-treatment of milk acidified with carbon-dioxide. J. Dairy Res 1992, 59, 95–99. [Google Scholar]
- Tomasula, P.M.; Boswell, R.T.; Dupre, N.C. Buffer properties of milk treated with high pressure carbon dioxide. Milchwissenschaft-Milk Sci. Int 1999, 54, 667–670. [Google Scholar]
- Tomasula, P.M.; Boswell, R.T. Measurement of the solubility of carbon dioxide in milk at high pressures. J. Supercrit. Fluid 1999, 16, 21–26. [Google Scholar]
- Jordan, P.J.; Lay, K.; Ngan, N.; Rodley, G.F. Casein precipitation using high pressure carbon dioxide. J. Dairy Sci. Technol. (N. Z.), 1987; 22, 247–256. [Google Scholar]
- Tomasula, P.M.; Craig, J.C., Jr; Boswell, R.T.; Cook, R.D.; Kurantz, M.J.; Maxwell, M. Preparation of casein using carbon dioxide. J. Dairy Sci 1995, 78, 506–514. [Google Scholar]
- Tomasula, P.M.; Craig, J.C., Jr; Boswell, R.T. A continuous process for casein production using high-pressure carbon dioxide. J. Food Eng 1997, 33, 405–419. [Google Scholar]
- Hofland, G.W.; Berkhoff, M.; Witkamp, G.J.; Van der Wielen, L.A.M. Dynamics of precipitation of casein with carbon dioxide. Int. Dairy J 2003, 13, 685–697. [Google Scholar]
- Hofland, G.W.; van Es, M.; van der Wielen, L.A.M.; Witkamp, G.J. Isoelectric precipitation of casein using high-pressure CO2. Ind. Eng. Chem. Res 1999, 38, 4919–4927. [Google Scholar]
- Dangaran, K.L.; Cooke, P.; Tomasula, P.M. The effect of protein particle size reduction on the physical properties of CO2-precipitated casein films. J. Food Sci 2006, 71, E196–E201. [Google Scholar]
- Kozempel, M.; Tomasula, P.M. Development of a semi-continuous process for CO2-precipitated-casein films. Abstr. Pap. Am. Chem. Soc 2005, 229, U302–U302. [Google Scholar]
- Tomasula, P.M.; Parris, N.; Yee, W.; Coffin, D. Properties of films made from CO2-precipitated casein. J. Agric. Food Chem 1998, 46, 4470–4474. [Google Scholar]
- Tomasula, P.M. Using dairy ingredients to produce edible films and biodegradable packaging materials. In Dairy-Derived Ingredients: Food and Nutraceutical Uses; Corredig, M., Ed.; Woodhead Publishing Ltd: Cambridge, UK, 2009; pp. 589–624. [Google Scholar]
- Tomasula, P.M.; Parris, N.; Boswell, R.T.; Moten, R.O. Preparation of enriched fractions of α-Lactalbumin and β-Lactoglobulin from cheese whey using carbon dioxide. J. Food Proc. Preserv 1998, 22, 463–476. [Google Scholar]
- Bonnaillie, L.M.; Tomasula, P.M.; Qi, P.X. Processes for Isolating Glycomacropeptides. U.S. Patent 13/211,689, 17 August 2011. [Google Scholar]
- Green, D.; Perry, R. Perry’s Chemical Engineers’ Handbook, 8th ed; McGraw-Hill: New York NY, USA, 2008. [Google Scholar]
- Parris, N.; White, A.E.; Farrell, H.M. Identification of altered proteins in nonfat dry milk powder prepared from heat-treated skim milk. J. Agric. Food Chem 1990, 38, 824–829. [Google Scholar]
- Lang, H. Cost relationships in preliminary cost estimation. Chem. Eng 1947, 54, 117–121. [Google Scholar]
- De Wit, J.N. Functional properties of whey proteins. In Developments in Dairy Chemistry—4; Fox, P.F., Ed.; Elsevier Applied Science: New York, NY, USA, 1989. [Google Scholar]
- Kinsella, J.E. Milk proteins: Physicochemical and functional properties. Crit. Rev. Food Sci. Nutr 1984, 21, 197–262. [Google Scholar]





| Composition solid fraction | Composition liquid fraction | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | T (°C) | CWPI % | P (MPa) | pH | Solid yield % | error * % | α- LA % | error * % | β- LG % | GMP % | minor % | α-LA recovery % | error * % | Purity ratio ** | β- LG % | β-LG recovery % | error * % | GMP % | GMP recovery % |
| 1 | 60 | 10 | 5.5 | 5.0 | 21.1 a | 1.7 | 53.9 | 1.5 | 28.0 | 5.5 | 12.6 | 63.3 a | 6.8 | 5.3 | - | 88.0a | 0.9 | - | 92.8 |
| 2 | 60 | 10 | 8.3 | 4.9 | 20.9 a | 2.5 | 51.9 | 2.1 | 28.2 | 4.2 | 15.8 | 60.1 a | 9.5 | 5.1 | 68.1 | 88.1a | 1.2 | 22.0 | 94.8 |
| 3 | 60 | 10 | 31.0 | 4.6 | 28.9 | 0.8 | 53.9 | 1.6 | 30.3 | 3.6 | 12.1 | 86.6 | 4.3 | 4.9 | 69.5 | 82.3 | 0.6 | 23.5 | 93.6 |
| 4 | 60 | 5 | 8.3 | 4.7 | 23.6 | 0.5 | 61.0 | 2.7 | 24.0 | 2.2 | 12.9 | 80.1 | 5.1 | 7.0 | - | 88.6 | 0.7 | - | 96.8 |
| 5 | 60 | 5 | 31.0 | 4.4 | 28.3 | 0.3 | 55.5 | 0.8 | 28.6 | 2.0 | 14.0 | 87.3 | 2.2 | 5.3 | 70.2 | 83.7 | 0.8 | 24.5 | 96.6 |
| 6 | 62 | 10 | 5.5 | 5.0 | 22.4 | 0.6 | 57.0 | 2.7 | 25.0 | 3.8 | 14.2 | 71.1 | 4.8 | 6.3 | 68.9 | 88.7 | 1.8 | 23.1 | 94.7 |
| 7 | 65 | 10 | 5.5 | 5.0 | 29.7 | 1.5 | 52.9 | 2.5 | 34.4 | 6.4 | 6.3 | 87.4 b | 0.2 | 4.2 | - | 79.3 | 2.3 | - | 88.3 |
| 8 | 65 | 10 | 8.3 | 4.9 | 35.0 | 0.1 | 44.7 | 3.3 | 38.8 | 4.4 | 12.1 | 86.9 b | 6.6 | 3.2 | - | 72.6 | 0.0 | - | 91.4 |
| 9 | 65 | 10 | 31.0 | 4.6 | 40.4 | 4.3 | 43.5 | 1.7 | 45.2 | 3.5 | 7.8 | 97.7 | 7.7 | 2.7 | 65.0 | 63.2 | 5.6 | 30.2 | 89.7 |
| 10 | 65 | 5 | 8.3 | 4.7 | 29.3 | 1.3 | 51.9 | 2.3 | 35.0 | 2.1 | 11.0 | 84.3 c | 0.1 | 4.1 | - | 79.3 | 2.8 | - | 96.2 |
| 11 | 65 | 5 | 31.0 | 4.4 | 36.5 | 2.2 | 38.8 | 1.1 | 49.4 | 2.3 | 9.5 | 78.7c | 6.5 | 2.2 | 64.1 | 63.6 | 2.7 | 28.7 | 95.3 |
| Flow rates (kg/h) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Water | 864.82 | 0 | 0 | 864.82 | 0 | 864.82 | 29.40 | 835.42 |
| Minerals | 0.91 | 0 | 0 | 0.91 | 0 | 0.91 | 0.03 | 0.88 |
| Lactose | 0.45 | 0 | 0 | 0.45 | 0 | 0.45 | 0.02 | 0.44 |
| α-LA | 8.16 | 0 | 0 | 8.16 | 0 | 8.16 | 7.13 | 1.04 |
| β-LG | 22.45 | 0 | 0 | 22.45 | 0 | 22.45 | 3.66 | 18.79 |
| GMP | 7.35 | 0 | 0 | 7.35 | 0 | 7.35 | 0.25 | 7.10 |
| Minor proteins | 2.86 | 0 | 0 | 2.86 | 0 | 2.86 | 1.69 | 1.17 |
| CO2 | 0.00 | 197.52 | 300.79 | 103.26 | 103.26 | 0 | 0.00 | 0.00 |
| Total | 907.00 | 197.52 | 300.79 | 1010.26 | 103.26 | 907.00 | 42.17 | 864.83 |
| Dry composition (w/w) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Minerals | 2.2% | 0.2% | 3.0% | |||||
| Lactose | 1.1% | 0.1% | 1.5% | |||||
| α-LA | 19.4% | 55.8% | 3.5% | |||||
| β-LG | 53.2% | 28.7% | 63.9% | |||||
| GMP | 17.4% | 2.0% | 24.1% | |||||
| Minor proteins | 6.8% | 13.2% | 4.0% | |||||
| CO2 | 0.0% | 0.0% | 0.0% | |||||
| Total | 100.0% | 100.0% | 100.0% |
| Name | Description | Specification | Cost (K$) |
|---|---|---|---|
| V-101 | Flat Bottom Tank | Volume = 1006.5 | L 28 |
| P-4 | Centrifugal Pump | Power = 0.04 | kW 9 |
| P-3 | Gear Pump | Power = 0.12 kW | 1 |
| V-103 | Stirred Reactor | Volume = 2.05 m3 | 1094 |
| HX-102 | Heat Exchanger | Area = 0.15 m2 | 1 |
| CFUGE | Disk-Stack Centrifuge | Throughput = 919 L/h | 450 |
| SDR-107 | Spray Dryer | Volume = 7.61 m3 | 897 |
| DDR-108 | Drum Dryer | Area = 1.36 m2 | 370 |
| G-106 | Centrifugal Compressor | Power = 99.8 | HP 400 |
| V-105 | Blending Tank | Volume = 0.46 m3 | 169 |
| Unlisted Equipment | 603 | ||
| TOTAL | 4022 |
| Case # | CWPI (wt %) | T (°C) | P (MPa) | pH | α-LA yield (wt %) | α-LA purity | α-LA recovery | Process cost ($/kg WPI) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 60 | 8.3 | 4.7 | 23.6% | 61.0% | 80.1% | 8.65 |
| 2 | 5 | 60 | 31.0 | 4.4 | 28.3% | 55.5% | 87.3% | 12.38 |
| 3 | 5 | 65 | 8.3 | 4.7 | 29.3% | 51.9% | 84.3% | 8.67 |
| 4 | 10 | 60 | 8.3 | 4.9 | 20.9% | 51.9% | 60.1% | 5.72 |
| 5 | 10 | 60 | 31.0 | 4.6 | 28.9% | 53.9% | 86.6% | 7.65 |
| 6 | 10 | 62 | 5.5 | 5.0 | 22.4% | 57.0% | 71.1% | 5.42 |
| 7 | 10 | 65 | 5.5 | 5.0 | 29.7% | 52.9% | 87.4% | 5.52 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yver, A.L.; Bonnaillie, L.M.; Yee, W.; McAloon, A.; Tomasula, P.M. Fractionation of Whey Protein Isolate with Supercritical Carbon Dioxide—Process Modeling and Cost Estimation. Int. J. Mol. Sci. 2012, 13, 240-259. https://doi.org/10.3390/ijms13010240
Yver AL, Bonnaillie LM, Yee W, McAloon A, Tomasula PM. Fractionation of Whey Protein Isolate with Supercritical Carbon Dioxide—Process Modeling and Cost Estimation. International Journal of Molecular Sciences. 2012; 13(1):240-259. https://doi.org/10.3390/ijms13010240
Chicago/Turabian StyleYver, Alexandra L., Laetitia M. Bonnaillie, Winnie Yee, Andrew McAloon, and Peggy M. Tomasula. 2012. "Fractionation of Whey Protein Isolate with Supercritical Carbon Dioxide—Process Modeling and Cost Estimation" International Journal of Molecular Sciences 13, no. 1: 240-259. https://doi.org/10.3390/ijms13010240
APA StyleYver, A. L., Bonnaillie, L. M., Yee, W., McAloon, A., & Tomasula, P. M. (2012). Fractionation of Whey Protein Isolate with Supercritical Carbon Dioxide—Process Modeling and Cost Estimation. International Journal of Molecular Sciences, 13(1), 240-259. https://doi.org/10.3390/ijms13010240