Self-Assembly of Discrete Metal Complexes in Aqueous Solution via Block Copolypeptide Amphiphiles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Copolypeptides
2.2. Spectroscopic Properties of Amphiphile/Metal Complex Composites
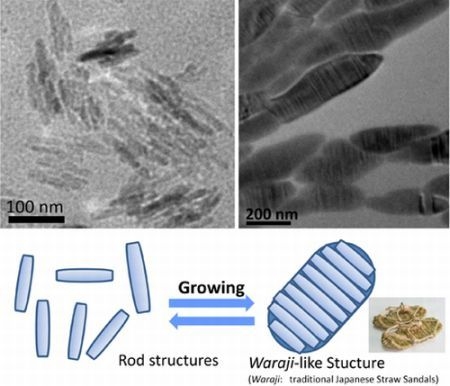
2.3. Morphological Characterization of Amphiphile/Metal Complex Composites
3. Experimental Section
3.1. Materials and Instrumentation
3.2. General Polypeptide Synthesis
3.3. General Preparation of Copolypeptide/[Au(CN)2]− Composites
4. Conclusions
Acknowledgments
References
- Suh, J.; Shim, H.; Shin, S. Coordinatively Polymerized Bilayer Membranes Prepared in Formamide. Langmuir 1996, 12, 2323–2324. [Google Scholar]
- Moulik, S.P.; De, G.C.; Panda, A.K.; Bhowmik, B.B.; Das, A.R. Dispersed Molecular Aggregates. 1. Synthesis and Characterization of Nanoparticles of Cu2[Fe(CN)6] in H2O/AOT/n-Heptane Water-in-Oil Microemulsion Media. Langmuir 1999, 15, 8361–8367. [Google Scholar]
- Vaucher, S.; Li, M.; Mann, S. Synthesis of Prussian Blue Nanoparticles and Nanocrystal Superlattices in Reverse Microemulsions. Angew. Chem. Int. Ed 2000, 39, 1793–1796. [Google Scholar]
- Vaucher, S.; Fielden, J.; Li, M.; Dujardin, E.; Mann, S. Molecule-Based Magnetic Nanoparticles: Synthesis of Cobalt Hexacyanoferrate, Cobalt Pentacyanonitrosylferrate, and Chromium Hexacyanochromate Coordination Polymers in Water-in-Oil Microemulsions. Nano Lett 2002, 2, 225–229. [Google Scholar]
- Uemura, T.; Kitagawa, S. Prussian Blue Nanoparticles Protected by Poly(vinylpyrrolidone). J. Am. Chem. Soc 2003, 125, 7814–7815. [Google Scholar]
- Uemura, T.; Ohba, M.; Kitagawa, S. Size and Surface Effects of Prussian Blue Nanoparticles Protected by Organic Polymers. Inorg. Chem 2004, 43, 7339–7345. [Google Scholar]
- Hu, M.; Furukawa, S.; Ohtani, R.; Sukegawa, H.; Nemoto, Y.; Reboul, J.; Kitagawa, S.; Yamauchi, Y. Synthesis of Prussian Blue Nanoparticles with a Hollow Interior by Controlled Chemical Etching. Angew. Chem. Int. Ed 2012, 51, 984–988. [Google Scholar]
- Domínguez-Vera, J.M.; Colacio, E. Nanoparticles of Prussian Blue Ferritin: A New Route for Obtaining Nanomaterials. Inorg. Chem 2003, 42, 6983–6985. [Google Scholar]
- Yamada, M.; Arai, M.; Kurihara, M.; Sakamoto, M.; Miyake, M. Synthesis and Isolation of Cobalt Hexacyanoferrate/Chromate Metal Coordination Nanopolymers Stabilized by Alkylamino Ligand with Metal Elemental Control. J. Am. Chem. Soc 2004, 126, 9482–9483. [Google Scholar]
- Fiorito, P.A.; Gonçales, V.R.; Ponzio, E.A.; de Torresi, S.I.C. Synthesis, characterization and immobilization of Prussian blue nanoparticles. A potential tool for biosensing devices. Chem. Commun. 2005, 366–368. [Google Scholar]
- Taguchi, M.; Yamada, K.; Suzuki, K.; Sato, O.; Einaga, Y. Photoswitchable Magnetic Nanoparticles of Prussian Blue with Amphiphilic Azobenzene. Chem. Mater 2005, 17, 4554–4559. [Google Scholar]
- Sun, X.; Dong, S.; Wang, E. Coordination-Induced Formation of Submicrometer-Scale, Monodisperse, Spherical Colloids of Organic-Inorganic Hybrid Materials at Room Temperature. J. Am. Chem. Soc 2005, 127, 13102–13103. [Google Scholar]
- Park, K.H.; Jang, K.; Son, S.U.; Sweigart, D.A. Self-Supported Organometallic Rhodium Quinonoid Nanocatalysts for Stereoselective Polymerization of Phenylacetylene. J. Am. Chem. Soc 2006, 128, 8740–8741. [Google Scholar]
- Oh, M.; Mirkin, C.A. Chemically tailorable colloidal particles from infinite coordination polymers. Nature 2005, 438, 651–654. [Google Scholar]
- Maeda, H.; Hasegawa, M.; Hashimoto, T.; Kakimoto, T.; Nishio, S.; Nakanishi, T. Nanoscale Spherical Architectures Fabricated by Metal Coordination of Multiple Dipyrrin Moieties. J. Am. Chem. Soc 2006, 128, 10024–10025. [Google Scholar]
- Imaz, I.; Maspoch, D.; Rodríguez-Blanco, C.; Pérez-Falcón, J.M.; Campo, J.; Ruiz-Molina, D. Valence-Tautomeric Metal–Organic Nanoparticles. Angew. Chem. Int. Ed 2008, 47, 1857–1860. [Google Scholar]
- Coronado, E.; Galán-Mascarós, J.R.; Monrabal-Capilla, M.; García-Martínez, J.; Pardo-Ibáñez, P. Bistable Spin-Crossover Nanoparticles Showing Magnetic Thermal Hysteresis near Room Temperature. Adv. Mater. 2007, 19, 1359–1361. [Google Scholar]
- Boldog, I.; Gaspar, A.B.; Martínez, V.; Pardo-Ibañez, P.; Ksenofontov, V.; Bhattacharjee, A.; Gütlich, P.; Real, J.A. Spin-Crossover Nanocrystals with Magnetic, Optical, and Structural Bistability near Room Temperature. Angew. Chem. Int. Ed. 2008, 47, 6433–6437. [Google Scholar]
- Liang, G.; Xu, J.; Wang, X. Synthesis and Characterization of Organometallic Coordination Polymer Nanoshells of Prussian Blue Using Miniemulsion Periphery Polymerization (MEPP). J. Am. Chem. Soc 2009, 131, 5378–5379. [Google Scholar]
- Rieter, W.J.; Taylor, K.M.L.; An, H.; Lin, W.; Lin, W. Nanoscale Metal-Organic Frameworks as Potential Multimodal Contrast Enhancing Agents. J. Am. Chem. Soc 2006, 128, 9024–9025. [Google Scholar]
- Taylor, K.M.L.; Rieter, W.J.; Lin, W. Manganese-Based Nanoscale Metal-Organic Frameworks for Magnetic Resonance Imaging. J. Am. Chem. Soc 2008, 130, 14358–14359. [Google Scholar]
- Jung, S.; Oh, M. Monitoring Shape Transformation from Nanowires to Nanocubes and Size-Controlled Formation of Coordination Polymer Particles. Angew. Chem. Int. Ed 2008, 47, 2049–2051. [Google Scholar]
- Rieter, W.J.; Pott, K.M.; Taylor, K.M.L.; Lin, W. Nanoscale Coordination Polymers for Platinum-Based Anticancer Drug Delivery. J. Am. Chem. Soc 2008, 130, 11584–11585. [Google Scholar]
- Suh, J.; Lee, K.J.; Bae, G.; Kwon, O.-B.; Oh, S. Coordinatively Polymerized Bilayer Membranes Prepared by Metal Complexation of an Amphiphilic o,o′-Dihydroxyazobenzene Derivative. Langmuir 1995, 11, 2626–2632. [Google Scholar]
- Bremi, J.; Brovelli, D.; Caseri, W.; Hähner, G.; Smith, P.; Tervoort, T. From Vauquelin’s and Magnus’ Salts to Gels, Uniaxially Oriented Films, and Fibers: Synthesis, Characterization, and Properties of Tetrakis(1-aminoalkane)metal(II) Tetrachlorometalates(II). Chem. Mater 1999, 11, 977–994. [Google Scholar]
- Grate, J.W.; Moore, L.K.; Janzen, D.E.; Veltkamp, D.J.; Kaganove, S.; Drew, S.M.; Mann, K.R. Steplike Response Behavior of a New Vapochromic Platinum Complex Observed with Simultaneous Acoustic Wave Sensor and Optical Reflectance Measurements. Chem. Mater 2002, 14, 1058–1066. [Google Scholar]
- Kurth, D.G.; Severin, N.; Rabe, J.P. Perfectly Straight Nanostructures of Metallosupramolecular Coordination- Polyelectrolyte Amphiphile Complexes on Graphite. Angew. Chem. Int. Ed 2002, 41, 3681–3683. [Google Scholar]
- Roubeau, O.; Colin, A.; Schmitt, V.; Clérac, R. Thermoreversible Gels as Magneto-Optical Switches. Angew. Chem. Int. Ed 2004, 43, 3283–3286. [Google Scholar]
- Kimizuka, N. Self-assembly of supramolecular nanofibers. Adv. Polym. Sci 2008, 219, 1–26. [Google Scholar]
- Kimizuka, N.; Oda, N.; Kunitake, T. Supramolecular assemblies comprised of one-dimensional mixed valence platinum complex and anionic amphiphiles in organic media. Chem. Lett 1998, 27, 695–696. [Google Scholar]
- Kuroiwa, K.; Shibata, T.; Takada, A.; Nemoto, N.; Kimizuka, N. Heat-set gel-like networks of lipophilic CoII triazole complexes in organic media and their thermochromic structural transitions. J. Am. Chem. Soc 2004, 126, 2016–2021. [Google Scholar]
- Kuroiwa, K.; Kimizuka, N. Coordination structure changes of linear cobalt(II) triazole complexes induced by binding of long-chained alcohols: Adaptive molecular clefts. Chem. Lett 2008, 37, 192–193. [Google Scholar]
- Kuroiwa, K.; Kimizuka, N. Electrochemically controlled self-assembly of lipophilic FeII 1,2,4-triazole complexes in organic media. Chem. Lett 2010, 39, 790–791. [Google Scholar]
- Kuroiwa, K.; Shibata, T.; Sasaki, S.; Ohba, M.; Takahara, A.; Kunitake, T.; Kimizuka, N. Supramolecular control of spin crossover phenomena in lipophilic FeII 1,2,4-triazole complexes. J. Polym. Sci. A Polym. Chem 2006, 44, 5192–5202. [Google Scholar]
- Kuroiwa, K.; Kikuchi, H.; Kimizuka, N. Spin crossover characteristics of nanofibrous FeII-1,2,4-triazole complexes in liquid crystals. Chem. Commun 2010, 46, 1229–1231. [Google Scholar]
- Yam, V.W.-W.; Cheng, E.C.-C. Highlights on the recent advances in gold chemistry—A photophysical perspective. Chem. Soc. Rev 2008, 37, 1806–1813. [Google Scholar]
- Wong, K.M.-C.; Yam, V.W.-W. Self-Assembly of Luminescent Alkynylplatinum(II) Terpyridyl Complexes: Modulation of Photophysical Properties through Aggregation Behavior. Acc. Chem. Res 2011, 44, 424–434. [Google Scholar]
- Kishimura, A.; Yamashita, T.; Aida, T. Phosphorescent Organogels via “Metallophilic” Interactions for Reversible RGB-Color Switching. J. Am. Chem. Soc 2005, 127, 179–183. [Google Scholar]
- Kishimura, A.; Yamashita, T.; Yamaguchi, K.; Aida, T. Rewritable phosphorescent paper by the control of competing kinetic and thermodynamic self-assembling events. Nat. Mater 2005, 4, 546–549. [Google Scholar]
- Mason, W.R. Electronic Structure and Spectra of Linear Dicyano Complexes. J. Am. Chem. Soc 1973, 95, 3573–3581. [Google Scholar]
- Rawashdeh-Omary, M.A.; Omary, M.A.; Patterson, H.H. Oligomerization of Au(CN)2− and Ag(CN)2− Ions in Solution via Ground-State Aurophilic and Argentophilic Bonding. J. Am. Chem. Soc 2000, 122, 10371–10380. [Google Scholar]
- Rawashdeh-Omary, M.A.; Omary, M.A.; Patterson, H.H.; Fackler, J.P., Jr. Excited-State Interactions for [Au(CN)2−]n and [Ag(CN)2−]n Oligomers in Solution. Formation of Luminescent Gold-Gold Bonded Excimers and Exciplexes. J. Am. Chem. Soc. 2001, 123, 11237–11247. [Google Scholar]
- Moriuchi, T.; Yoshii, K.; Katano, C.; Hirao, T. Poly-L-lysine-induced Self-association and Luminescence of Dicyanoaurate(I). Chem. Lett 2010, 39, 841–843. [Google Scholar]
- Pochan, D.J.; Pakstis, L.; Ozbas, B.; Nowak, A.P.; Deming, T.J. SANS and cryo-TEM Study of Self-assembled Diblock Copolypeptide Hydrogels with Rich Nano-through Microscale Morphology. Macromolecules 2002, 35, 5358–5360. [Google Scholar]
- Novak, A.P.; Breedveld, V.; Pakstis, L.; Ozbas, B.; Pine, D.J.; Pochan, D.; Deming, T.J. Rapidly Recovering Hydrogel Scaffolds from Self-assembling Diblock Copolypeptide Amphiphiles. Nature 2002, 417, 424–428. [Google Scholar]
- Deming, T.J. Methodologies for Preparation of Synthetic Block Copolypeptides: Materials with Future Promise in Drug Deliverly. Adv. Drug Deliv. Rev 2002, 54, 1145–1155. [Google Scholar]
- Breedveld, V.; Nowak, A.P.; Sato, J.; Deming, T.J.; Pine, D.J. Rheology of Block Copolypeptide Solutions: Hydrogels with tunable properties. Macromolecules 2004, 37, 3943–3953. [Google Scholar]
- Deming, T.J. Polypeptide Hydrogels via a Unique Assembly Mechanism. Soft Matter 2005, 1, 28–35. [Google Scholar]
- Holowka, E.P.; Sun, V.Z.; Kamei, D.T.; Deming, T.J. Polyarginine Segments in Block Copolypeptides Drive both Vesicular Assembly and Intracellular Delivery. Nat. Mater 2007, 6, 52–57. [Google Scholar]
- Yang, C.-Y.; Song, B.; Ao, Y.; Nowak, A.P.; Abelowitz, R.B.; Korsak, R.A.; Havton, L.A.; Deming, T.J.; Sofroniew, M.V. Biocompatibility of Amphiphilic Diblock Copolypeptide Hydrogels in the Central Nervous System. Biomaterials 2009, 30, 2881–2898. [Google Scholar]
- Tomczak, M.M.; Glawe, D.D.; Drummy, L.F.; Lawrence, C.G.; Stone, M.O.; Perry, C.C.; Pochan, D.J.; Deming, T.J.; Naik, R.R. Polypeptide-Templated Synthesis of Hexagonal Silica Platelets. J. Am. Chem. Soc 2005, 127, 12577–12582. [Google Scholar]
- Bellomo, E.G.; Deming, T.J. Monoliths of Aligned Silica-Polypeptide Hexagonal Platelets. J. Am. Chem. Soc 2006, 128, 2276–2279. [Google Scholar]
- Higuchi, T.; Tajima, A.; Yabu, H.; Shimomura, M. Spontaneous Formation of Polymer Nanoparticles with Inner Micro-Phase Separation Structures. Soft Matter 2008, 4, 1302–1305. [Google Scholar]
- Yabu, H.; Koike, K.; Motoyoshi, K.; Higuchi, T.; Shimomura, M. A Novel Route for Fabricating Metal-Polymer Composite Nanoparticles with Phase-Separated Structures. Macromol. Rapid Commun 2010, 31, 1267–1271. [Google Scholar]
- Camerel, F.; Strauch, P.; Antonietti, M.; Faul, C.F.J. Copper-Metallomesogen Structures Obtained by Ionic Self-Assembly (ISA): Molecular Electromechanical Switching Driven by Cooperativity. Chem. Eur. J 2003, 9, 3764–3771. [Google Scholar]
- Kuroiwa, K.; Yoshida, M.; Masaoka, S.; Kaneko, K.; Sakai, K.; Kimizuka, N. Self-Assembly of Tubular Microstructures from Mixed-Valence Metal Compolexes and Their Reversible Transformation by External Stimuli. Angew. Chem. Int. Ed 2012, 51, 656–659. [Google Scholar]









© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kuroiwa, K.; Masaki, Y.; Koga, Y.; Deming, T.J. Self-Assembly of Discrete Metal Complexes in Aqueous Solution via Block Copolypeptide Amphiphiles. Int. J. Mol. Sci. 2013, 14, 2022-2035. https://doi.org/10.3390/ijms14012022
Kuroiwa K, Masaki Y, Koga Y, Deming TJ. Self-Assembly of Discrete Metal Complexes in Aqueous Solution via Block Copolypeptide Amphiphiles. International Journal of Molecular Sciences. 2013; 14(1):2022-2035. https://doi.org/10.3390/ijms14012022
Chicago/Turabian StyleKuroiwa, Keita, Yoshitaka Masaki, Yuko Koga, and Timothy J. Deming. 2013. "Self-Assembly of Discrete Metal Complexes in Aqueous Solution via Block Copolypeptide Amphiphiles" International Journal of Molecular Sciences 14, no. 1: 2022-2035. https://doi.org/10.3390/ijms14012022
APA StyleKuroiwa, K., Masaki, Y., Koga, Y., & Deming, T. J. (2013). Self-Assembly of Discrete Metal Complexes in Aqueous Solution via Block Copolypeptide Amphiphiles. International Journal of Molecular Sciences, 14(1), 2022-2035. https://doi.org/10.3390/ijms14012022