Synthesis and Self-Organization of Fluorene-Conjugated Bisimidazolylporphyrin and Its Optical Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
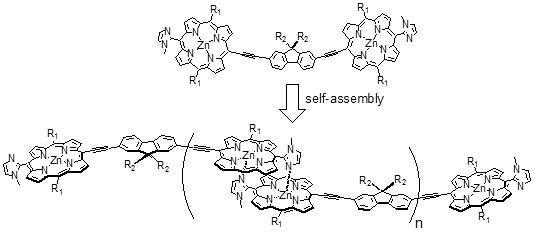
2.2. Formation of Self-Assembled Polymer
2.3. UV-Vis Absorption Spectra
2.4. Nonlinear Absorption
3. Experimental Section
General
4. Conclusions
Acknowledgments
References
- Gunes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev 2007, 107, 1324–1338. [Google Scholar]
- Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev 2009, 109, 897–1091. [Google Scholar]
- Irie, M. Photochromism: Memories and switches—Introduction. Chem. Rev 2000, 100, 1683–1683. [Google Scholar]
- Drobizhev, M.; Stepanenko, Y.; Rebane, A.; Wilson, C.J.; Screen, T.E.O.; Anderson, H.L. Strong cooperative enhancement of two-photon absorption in double-strand conjugated porphyrin ladder arrays. J. Am. Chem. Soc 2006, 128, 12432–12433. [Google Scholar]
- Drobizhev, M.; Stepanenko, Y.; Dzenis, Y.; Karotki, A.; Rebane, A.; Taylor, P.N.; Anderson, H.L. Understanding strong two-photon absorption in pi-conjugated porphyrin dimers via double-resonance enhancement in a three-level model. J. Am. Chem. Soc 2004, 126, 15352–15353. [Google Scholar]
- Hisaki, I.; Hiroto, S.; Kim, K.S.; Noh, S.B.; Kim, D.; Shinokubo, H.; Osuka, A. Synthesis of doubly beta-to-beta 1,3-butadiyne-bridged diporphyrins: Enforced planar structures and large two-photon absorption cross sections. Angew. Chem. Int. Ed 2007, 46, 5125–5128. [Google Scholar]
- Ikeda, C.; Yoon, Z.S.; Park, M.; Inoue, H.; Kim, D.; Osuka, A. Helicity induction and two-photon absorbance enhancement in zinc(ii) meso-meso linked porphyrin oligomers via intermolecular hydrogen bonding interactions. J. Am. Chem. Soc 2005, 127, 534–535. [Google Scholar]
- Bhaskar, A.; Guda, R.; Haley, M.M.; Goodson, T. Building symmetric two-dimensional two-photon materials. J. Am. Chem. Soc 2006, 128, 13972–13973. [Google Scholar]
- Bhaskar, A.; Ramakrishna, G.; Lu, Z.K.; Twieg, R.; Hales, J.M.; Hagan, D.J.; van Stryland, E.; Goodson, T. Investigation of two-photon absorption properties in branched alkene and alkyne chromophores. J. Am. Chem. Soc 2006, 128, 11840–11849. [Google Scholar]
- Arnbjerg, J.; Jimenez-Banzo, A.; Paterson, M.J.; Nonell, S.; Borrell, J.I.; Christiansen, O.; Ogilby, P.R. Two-photon absorption in tetraphenylporphycenes: Are porphycenes better candidates than porphyrins for providing optimal optical properties for two-photon photodynamic therapy. J. Am. Chem. Soc 2007, 129, 5188–5199. [Google Scholar]
- Kamada, K.; Ohta, K.; Kubo, T.; Shimizu, A.; Morita, Y.; Nakasuji, K.; Kishi, R.; Ohta, S.; Furukawa, S.; Takahashi, H.; et al. Strong two-photon absorption of singlet diradical hydrocarbons. Angew. Chem. Int. Ed 2007, 46, 3544–3546. [Google Scholar]
- Ogawa, K.; Zhang, T.Q.; Yoshihara, K.; Kobuke, Y. Large third-order optical nonlinearity of self-assembled porphyrin oligomers. J. Am. Chem. Soc 2002, 124, 22–23. [Google Scholar]
- Ogawa, K.; Ohashi, A.; Kobuke, Y.; Kamada, K.; Ohta, K. Strong two-photon absorption of self-assembled butadiyne-linked bisporphyrin. J. Am. Chem. Soc 2003, 125, 13356–13357. [Google Scholar]
- Ogawa, K.; Ohashi, A.; Kobuke, Y.; Kamada, K.; Ohta, K. Two-photon absorption properties of self-assemblies of butadiyne-linked bis(imidazolylporphyrin). J. Phys. Chem. B 2005, 109, 22003–22012. [Google Scholar]
- Ogawa, K.; Hasegawa, H.; Inaba, Y.; Kobuke, Y.; Inouye, H.; Kanemitsu, Y.; Kohno, E.; Hirano, T.; Ogura, S.; Okura, I. Water-soluble bis(imidazolylporphyrin) self-assemblies with large two-photon absorption cross sections as potential agents for photodynamic therapy. J. Med. Chem 2006, 49, 2276–2283. [Google Scholar]
- Tanihara, J.; Ogawa, K.; Kobuke, Y. Two-photon absorption properties of conjugated supramolecular porphyrins with electron donor and acceptor. J. Photochem. Photobiol. A 2006, 178, 140–149. [Google Scholar]
- Dy, J.T.; Ogawa, K.; Satake, A.; Ishizumi, A.; Kobuke, Y. Water-soluble self-assembled butadiyne-bridged bisporphyrin: A potential two-photon-absorbing photosensitizer for photodynamic therapy. Chem. Eur. J 2007, 13, 3491–3500. [Google Scholar]
- Dy, J.T.; Maeda, R.; Nagatsuka, Y.; Ogawa, K.; Kamada, K.; Ohta, K.; Kobuke, Y. A photochromic porphyrin-perinaphthothioindigo conjugate and its two-photon absorption properties. Chem. Commun. 2007, 5170–5172. [Google Scholar]
- Dy, J.; Ogawa, K.; Kamada, K.; Ohta, K.; Kobuke, Y. Stepwise elongation effect on the two-photon absorption of self-assembled butadiyne porphyrins. Chem. Commun. 2008, 3411–3413. [Google Scholar]
- Raymond, J.E.; Bhaskar, A.; Goodson, T.; Makiuchi, N.; Ogawa, K.; Kobuke, Y. Synthesis and two-photon absorption enhancement of porphyrin macrocycles. J. Am. Chem. Soc 2008, 130, 17212–17213. [Google Scholar]
- Morisue, M.; Ogawa, K.; Kamada, K.; Ohta, K.; Kobuke, Y. Strong two-photon and three-photon absorptions in the antiparallel dimer of a porphyrin-phthalocyanine tandem. Chem. Commun 2010, 46, 2121–2123. [Google Scholar]
- Kamada, K.; Hara, C.; Ogawa, K.; Ohta, K.; Kobuke, Y. Strong two-photon absorption and its saturation of a self-organized dimer of an ethynylene-linked porphyrin tandem. Chem. Commun 2012, 48, 7988–7990. [Google Scholar]
- Kobuke, Y.; Miyaji, H. Supramolecular organization of imidazolyl-porphyrin to a slipped cofacial dimer. J. Am. Chem. Soc 1994, 116, 4111–4112. [Google Scholar]
- Kobuke, Y.; Ogawa, K. Porphyrin supramolecules for artificial photosynthesis and molecular photonic/electronic materials. Bull. Chem. Soc. Jpn 2003, 76, 689–708. [Google Scholar]
- Ogawa, K.; Kobuke, Y. Formation of a giant supramolecular porphyrin array by self-coordination. Angew. Chem. Int. Ed 2000, 39, 4070–4073. [Google Scholar]
- Rogers, J.E.; Slagle, J.E.; McLean, D.G.; Sutherland, R.L.; Brant, M.C.; Heinrichs, J.; Jakubiak, R.; Kannan, R.; Tan, L.S.; Fleitz, P.A. Insight into the nonlinear absorbance of two related series of two-photon absorbing chromophores. J. Phys. Chem. A 2007, 111, 1899–1906. [Google Scholar]
- Morales, A.R.; Belfield, K.D.; Hales, J.M.; Van Stryland, E.W.; Hagan, D.J. Synthesis of two-photon absorbing unsymmetrical fluorenyl-based chromophores. Chem. Mater 2006, 18, 4972–4980. [Google Scholar]
- Padmawar, P.A.; Rogers, J.E.; He, G.S.; Chiang, L.Y.; Tan, L.S.; Canteenwala, T.; Zheng, Q.D.; Slagle, J.E.; McLean, D.G.; Fleitz, P.A.; et al. Large cross-section enhancement and intramolecular energy transfer upon multiphoton absorption of hindered diphenylaminofluorene-c-60 dyads and triads. Chem. Mater 2006, 18, 4065–4074. [Google Scholar]
- Zheng, Q.D.; He, G.S.; Prasad, P.N. Pi-conjugated dendritic nanosized chromophore with enhanced two-photon absorption. Chem. Mater 2005, 17, 6004–6011. [Google Scholar]
- Belfield, K.D.; Morales, A.R.; Kang, B.S.; Hales, J.M.; Hagan, D.J.; van Stryland, E.W.; Chapela, V.M.; Percino, J. Synthesis, characterization, and optical properties of new two-photon-absorbing fluorene derivatives. Chem. Mater 2004, 16, 4634–4641. [Google Scholar]
- Belfield, K.D.; Hagan, D.J.; van Stryland, E.W.; Schafer, K.J.; Negres, R.A. New two-photon absorbing fluorene derivatives: Synthesis and nonlinear optical characterization. Org. Lett 1999, 1, 1575–1578. [Google Scholar]
- Reinhardt, B.A.; Brott, L.L.; Clarson, S.J.; Dillard, A.G.; Bhatt, J.C.; Kannan, R.; Yuan, L.X.; He, G.S.; Prasad, P.N. Highly active two-photon dyes: Design, synthesis, and characterization toward application. Chem. Mater 1998, 10, 1863–1874. [Google Scholar]
- Yao, S.; Belfield, K.D. Synthesis of two-photon absorbing unsymmetrical branched chromophores through direct tris(bromomethylation) of fluorene. J. Org. Chem 2005, 70, 5126–5132. [Google Scholar]
- Susumu, K.; Duncan, T.V.; Therien, M.J. Potentiometric, electronic structural, and ground- and excited-state optical properties of conjugated bis[(porphinato)zinc(ii)] compounds featuring proquinoidal spacer units. J. Am. Chem. Soc 2005, 127, 5186–5195. [Google Scholar]
- Lee, S.H.; Nakamura, T.; Tsutsui, T. Synthesis and characterization of oligo(9,9-dihexyl-2,7-fluorene ethynylene)s: For application as blue light-emitting diode. Org. Lett 2001, 3, 2005–2007. [Google Scholar]
- Kannan, R.; He, G.S.; Lin, T.C.; Prasad, P.N.; Vaia, R.A.; Tan, L.S. Toward highly active two-photon absorbing liquids. Synthesis and characterization of 1,3,5-triazine-based octupolar molecules. Chem. Mater 2004, 16, 185–194. [Google Scholar]
- Balke, S.T.; Hamielec, A.E.; LeClair, B.P.; Pearce, S.L. Gel permeation chromatography. Ind. Eng. Chem. Prod. Res. Dev 1969, 8, 54–57. [Google Scholar]
- Kamada, K.; Ohta, K.; Yoichiro, I.; Kondo, K. Two-photon absorption properties of symmetric substituted diacetylene: Drastic enhancement of the cross section near the one-photon absorption peak. Chem. Phys. Lett 2003, 372, 386–393. [Google Scholar]
- Drobizhev, M.; Karotki, A.; Kruk, M.; Rebane, A. Resonance enhancement of two-photon absorption in porphyrins. Chem. Phys. Lett 2002, 355, 175–182. [Google Scholar]






© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ogawa, K.; Makiuchi, N.; Kobuke, Y. Synthesis and Self-Organization of Fluorene-Conjugated Bisimidazolylporphyrin and Its Optical Properties. Int. J. Mol. Sci. 2013, 14, 322-331. https://doi.org/10.3390/ijms14010322
Ogawa K, Makiuchi N, Kobuke Y. Synthesis and Self-Organization of Fluorene-Conjugated Bisimidazolylporphyrin and Its Optical Properties. International Journal of Molecular Sciences. 2013; 14(1):322-331. https://doi.org/10.3390/ijms14010322
Chicago/Turabian StyleOgawa, Kazuya, Naoyuki Makiuchi, and Yoshiaki Kobuke. 2013. "Synthesis and Self-Organization of Fluorene-Conjugated Bisimidazolylporphyrin and Its Optical Properties" International Journal of Molecular Sciences 14, no. 1: 322-331. https://doi.org/10.3390/ijms14010322
APA StyleOgawa, K., Makiuchi, N., & Kobuke, Y. (2013). Synthesis and Self-Organization of Fluorene-Conjugated Bisimidazolylporphyrin and Its Optical Properties. International Journal of Molecular Sciences, 14(1), 322-331. https://doi.org/10.3390/ijms14010322