Why Flavins Are not Competitors of Chlorophyll in the Evolution of Biological Converters of Solar Energy
Abstract
:1. Introduction
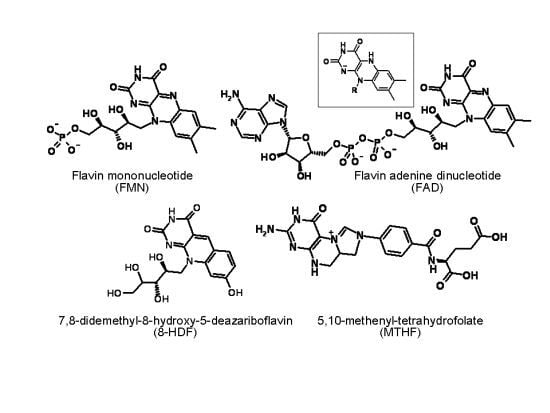
2. Flavins Are Evolutionarily Ancient Molecules
3. Flavin Molecules Display Photochemical Activity
4. Flavin Can Convert Light Energy into the Energy of ATP: A Prebiotic Process Model
5. Biological Evolution Repeatedly Chose Flavins for Light Receptors
6. How Do Excited Flavins Exhibit Chemical Activity in Photoreceptor Proteins?
7. Evolution Has Equipped Some Flavin Photoreceptors with Light-Harvesting Antennae
8. The Environmental Framework for a Hypothetical Energy Converter
9. Could Flavin Compete with Chlorophyll in the Evolution of Solar Energy Converters?
10. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Schopf, J.W. Fossil evidence of Archaean life. Phil. Trans. R. Soc. B 2006, 361, 869–885. [Google Scholar]
- Blankenship, R.E. Early evolution of photosynthesis. Plant Physiol 2010, 154, 434–438. [Google Scholar]
- Whitmarsh, J.; Govindjee. The Photosynthetic Process. In Concepts in Photobiology: Photosynthesis and Photomorphogenesis; Singhal, G.S., Renger, G., Eds.; Narosa Publishers: New Delhi, India; Kluwer Academic: Dordrecht, The Netherlands, 1999; pp. 11–51. [Google Scholar]
- Osterhelt, D. The structure and mechanism of the family of retinal proteins from halophilic Archaea. Curr. Opin. Struct. Biol 1998, 8, 489–500. [Google Scholar]
- Fischer, M.; Schott, A.K.; Romisch, W.; Ramsperger, A.; Augustin, M.; Fidler, A.; Bacher, A.; Richter, G.; Huber, R.; Eisenreich, W. Evolution of vitamin B2 biosynthesis. A novel class of riboflavin synthase in Archaea. J. Mol. Biol 2004, 343, 267–278. [Google Scholar]
- Zylberman, V.; Klinke, S.; Haase, I.; Bacher, A.; Fisher, M.; Goldbaum, F.A. Evolution of vitamin B2 biosynthesis: 6,7-dimethyl-8-ribityllumazine synthases of Brucella. J. Bacteriol 2006, 188, 6135–6142. [Google Scholar]
- Heinz, B.; Ried, W.; Dose, K. Thermische erzeugung von pteridinen und flavinen aus aminosäueregemischen. Angew. Chem 1979, 91, 510–511. [Google Scholar]
- Heinz, B.; Ried, W. The formation of chromophores through amino acid thermolysis and their possible role as prebiotic photoreceptors. BioSystems 1981, 14, 33–40. [Google Scholar]
- Kolesnikov, M.P.; Kritsky, M.S. Study of chemical structure and of photochemical activity of abiogenic flavin pigment. J. Evol. Biochem. Physiol 2001, 37, 507–514. [Google Scholar]
- Fox, S.W.; Dose, K. Molecular Evolution and the Origin of Life; S.A. Freeman Co.: San Francisco, CA, USA, 1972; p. 359. [Google Scholar]
- Lai, E.C. RNA sensors and riboswitches: Self-regulating messages. Curr. Biol 2003, 13, R285–R291. [Google Scholar]
- Kritsky, M.S.; Telegina, T.A. Role of Nucleotide-Like Coenzymes in Primitive Evolution. Role of Nucleotide-Like Coenzymes in Primitive Evolution. In Origins: Genesis, Evolution and Diversity of Life; Seckbach, J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 215–231. [Google Scholar]
- Heelis, P.F. The photophysical and photochemical properties of flavins (izoalloxazines). Chem. Soc. Rev 1982, 11, 15–39. [Google Scholar]
- Massey, V. The chemical and biological versatility of riboflavin. Biochem. Soc. Trans 2000, 28, 283–296. [Google Scholar]
- Hemmerich, P.; Schmidt, W. Bluelight Reception and Flavin Photochemistry. In Photoreception and Sensory Transduction in Aneural Organisms; Lenci, F., Colombetti, G., Eds.; Plenum Press: New York, NY, USA, 1979; pp. 341–354. [Google Scholar]
- Knappe, W.R.; Hemmerich, P. Covalent intermediates in flavin-sensitized photodehydrogenation and photodecarboxylation. Z. Naturforsrh. Teil B 1972, 27, 1022–1035. [Google Scholar]
- Vincent, V.; Stankovich, M.; Hemmerich, P. Light-mediated reduction of flavoproteins with flavins as catalysts. Biochemistry 1978, 17, 1–8. [Google Scholar]
- Holmstrom, B. Flash photoreduction of flavin mononucleotide in neutral solutions. Absolute absorptivity of the semiquinone. Photochem. Photobiol 1964, 3, 97–114. [Google Scholar]
- Vaish, S.P.; Tollin, G. Flash photolysis of flavins. IV. Some properties of the lumiflavin triplet state. Bioenergetics 1970, 1, 181–192. [Google Scholar]
- Vaish, S.P.; Tollin, G. Flash photolysis of flavins. V. Oxidation and disproportionation of flavin radicals. J. Bioenerg 1971, 2, 61–72. [Google Scholar]
- Brüstlein, M.; Knappe, W.R.; Hemmerich, P. Novel photoalkylation reactions on the flavin nucleus. Angew. Chem. Int. Ed. Engl 1971, 10, 804–806. [Google Scholar]
- Hemmerich, P.; Haas, W. Recent Developments in the Study of “Fully Reduced Flavin”. In Reactivity of Flavins; Yagi, K., Ed.; University of Tokyo Press: Tokyo, Japan, 1975; pp. 1–13. [Google Scholar]
- Heelis, P.F.; Hartman, R.F.; Rose, S.D. Photoenzymic repair of UV-damaged DNA: A chemist’s perspective. Chem. Soc. Rev 1995, 24, 289–297. [Google Scholar]
- Egorov, S.Y.; Krasnovsky, A.A., Jr; Bashtanov, M.Y.; Mironov, E.A.; Ludnikova, T.A.; Kritsky, M.S. Photosensitization of singlet oxygen formation by pterins and flavins. Time-resolved studies of oxygen phosphorescence under laser excitation. Biochemistry-Moscow 1999, 64, 1117–1121. [Google Scholar]
- Baier, J.; Maisch, T.; Maier, M.; Engel, E.; Landthaler, M.; Bäumler, W. Singlet oxygen generation by UVA light exposure of endogenous photosensitizers. Biophys. J 2006, 91, 1452–1459. [Google Scholar]
- Krasnovskii, A.A., Jr; Chernysheva, E.K.; Kritskii, M.S. Investigation of the role of active forms of oxygen in flavin-photosensitized oxidation of NADH. Biochemistry-Moscow 1987, 52, 1273–1282. [Google Scholar]
- Acworth, I.N. The Handbook of Redox Biochemistry; ESA Inc.: Chelmsford, MA, USA, 2003; p. 157. [Google Scholar]
- Frisell, W.R.; Chung, C.W.; Mackenzie, C.G. Catalysis of oxidation of nitrogen compounds by flavin coenzymes in the presence of light. J. Biol. Chem 1959, 234, 1297–1302. [Google Scholar]
- Vernon, L.P. Photochemical oxidation and reduction reactions catalyzed by flavin nucleotides. Biochim. Biophys. Acta 1959, 36, 177–185. [Google Scholar]
- Song, S.-H.; Dick, B.; Penzkofer, A. Photo-induced reduction of flavin mononucleotide in aqueous solutions. Chem. Phys 2007, 332, 55–65. [Google Scholar]
- Bladel, W.T.; Laessig, R.H. Continous EDTA titrations with a dropping mercury electrode. Automated titrations based on nonsymmetric curves. Anal. Chem 1965, 37, 1255–1260. [Google Scholar]
- Schmidt, W.; Butler, W.L. Flavin-mediated photoreactions in artificial systems—Possible model for blue-light photoreceptor pigment in living systems. Photochem 1976, 24, 71–76. [Google Scholar]
- Kolesnikov, M.P.; Telegina, T.A.; Lyudnikova, T.A.; Kritsky, M.S. Abiogenic photophosphoryation of ADP to ATP sensitized by flavoproteinoid microspheres. Orig. Life Evol. Biosph 2008, 38, 243–255. [Google Scholar]
- Kolesnikov, M.P. Proteinoid microspheres and the process of prebiological photophosphorylation. Orig. Life Evol. Biosph 1991, 21, 31–37. [Google Scholar]
- Kritsky, M.S.; Kolesnikov, M.P.; Telegina, T.A. Modeling of abiogenic synthesis of ATP. Doklady Biochem. Biophys 2007, 417, 313–315. [Google Scholar]
- Svoboda, J.; König, B. Templated photochemistry: Toward catalysts enhancing the efficiency and selectivity of photoreactions in homogeneous solutions. Chem. Rev 2006, 106, 5413–5430. [Google Scholar]
- Telegina, T.A.; Kolesnikov, M.P.; Vechtomova, Y.L.; Kritsky, M.S. Abiotic photophosphorylation model based on abiogenic flavin and pteridine pigments. 2012. Submitted to publication. [Google Scholar]
- Losinova, T.A.; Nedelina, O.S.; Kayushin, L.P. Effect of adenosine diphosphate on the light-dependent oxygen absorption by flavins. Biophysics 1986, 31, 10–15. [Google Scholar]
- Nedelina, O.S. Biosynthesis of ATP. In Elementary Chemical Act of ATP Synthesis in Oxidative Phosphorylation; Institute of Biochemical Physics RAS: Moscow, Russia, 1997; p. 304. [Google Scholar]
- Maheen, G.; Tian, G.; Wang, Y.; He, C.; Shi, Z.; Yuan, H.; Feng, S. Mimicking the prebiotic acidic hydrothermal environment: One-pot prebiotic hydrothermal synthesis of glucose phosphates. Heteroat. Chem 2011, 22, 186–191. [Google Scholar]
- Krasnovskii, A.A. Development of the Mode of Action of the Photocatalytic System in Organisms; Proceedings of 1st International Symposium on the Origin of Life on the Earth, Moscow, Russia, 19–24 August 1957, Oparin, A.I., Pasynskii, A.G., Braunstein, A.E., Pavlovskaya, T.E., Clark, F., Synge, R.L.M., Eds.; Pergamon Press: London, UK, 1959; pp. 606–618. [Google Scholar]
- Sancar, A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem. Rev 2003, 103, 2203–2223. [Google Scholar]
- Sancar, A. Structure and function of photolyase and in vivo enzymology: 50th anniversary (minireview). J. Biol. Chem 2008, 283, 32153–32157. [Google Scholar]
- Partch, C.L.; Sancar, A. Photochemistry and photobiology of cryptochrome blue-light photopigments: The search for a photocycle. Photochem. Photobiol 2005, 81, 1291–1304. [Google Scholar]
- Müller, M.; Carell, T. Structural biology of DNA photolyases and cryptochromes. Curr. Opin. Struct. Biol 2009, 19, 277–285. [Google Scholar]
- Losi, A. Flavin-based blue-light photosensors: A photobiophysics update. Photochem. Photobiol 2007, 83, 1283–1300. [Google Scholar]
- Losi, A.; Gaertner, W. Old chromophores, new photoactivation paradigms, trendy applications: Flavins in blue light-sensing photoreceptors. Photochem. Photobiol 2011, 87, 491–510. [Google Scholar]
- Metz, S.; Jaeger, A.; Klug, G. In vivo sensitivity of blue-light-dependent signaling mediated by AppA/PpsR or PrrB/PrrA in Rhodobacter sphaeroides. J. Bacteriol 2009, 191, 4473–4477. [Google Scholar]
- Schafmeier, T.; Diernfellner, A.C.R. Light input and processing in the circadian clock of Neurospora. FEBS Lett 2011, 585, 1467–1473. [Google Scholar]
- Swartz, T.E.; Tseng, T.-S.; Frederickson, M.A.; Paris, G.; Comerci, D.J.; Rajashekara, G.; Kim, J.-G.; Mudgett, M.B.; Splitter, G.A.; Ugalde, R.A.G.; et al. Blue-light-activated histidine kinases: Two-component sensors in bacteria. Science 2007, 317, 1090–1093. [Google Scholar]
- Barends, T.R.M.; Hartmann, E.; Griese, J.J.; Beitlich, T.; Kirienko, N.; Ryjenkov, D.; Reinstein, J.; Shoeman, R.; Gomelsky, M.; Schlichting, I. Structure and mechanism of a bacterial light-regulated cyclic nucleotide phosphodiesterase. Nature 2009, 459, 1015–1018. [Google Scholar]
- Ito, S.; Murakami, A.; Iseki, M.; Takahashi, T.; Higashi, S.; Watanabe, M. Differentiation of photocycle characteristics of flavin-binding BLUF domains of α-β-subunits of photoactivated adenylyl cyclase of Euglena gracilis. Photochem. Photobiol. Sci. 2010, 9, 1327–1335. [Google Scholar]
- Kritsky, M.S.; Telegina, T.A.; Vechtomova, Y.L.; Kolesnikov, M.P.; Lyudnikova, T.A.; Golub, O.A. Excited flavin and pterin coenzyme molecules in evolution. Biochemistry 2010, 75, 1200–1216. [Google Scholar]
- Corrochano, L.M. Fungal photoreceptors: Sensory molecules for fungal development and behaviour. Photochem. Photobiol. Sci 2007, 6, 725–736. [Google Scholar]
- Gauden, M.; Stokkum, I.H.M.; van Ihalainen, J.A.; Grondelle, R.; van Kennis, J.T.M.; Yeremenko, S.; Laan, W.; Hellingwerf, K.J. Photocycle of the flavin-binding photoreceptor APPA, a bacterial transcriptional antirepressor of photosynthesis genes. Biochemistry 2005, 44, 3653–3662. [Google Scholar]
- Dragnea, V.; Waegle, M.; Balascuta, S.; Bauer, C.; Dragnea, B. Time-resolved spectroscopic studies of the AppA blue-light receptor BLUF domain from Rhodobacter sphaeroides. Biochemistry 2005, 44, 15978–15985. [Google Scholar]
- Salomon, M.; Christie, J.M.; Knieb, E.; Lempert, U.; Briggs, W.R. Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry 2000, 39, 9401–9410. [Google Scholar]
- Stokkum, I.H.M.; van Gauden, M.; Crosson, S.; Grondelle, R.; van Moffat, K.; Kennis, J.T.M. The primary photophysics of the Avena sativa phototropin 1 LOV2 domain observed with time-resolved emission spectroscopy. Photochem. Photobiol 2011, 87, 534–541. [Google Scholar]
- Brudler, R.; Hitomi, K.; Diayasu, H.; Toh, H.; Kucho, K.; Ishiura, M.; Kanehisa, M.; Roberts, V.A.; Todo, T.; Tainer, J.A.; et al. Identification of a new cryptochrome class. Structure, function, and evolution. Mol. Cell 2003, 11, 59–67. [Google Scholar]
- Swartz, T.E.; Corchnoy, S.B.; Christie, J.M.; Lewis, J.L.; Schundi, W.R.; Briggs, W.R.; Bogomolni, R.A. The photocycle of flavin binding domain of the blue light photoreceptor phototropin. J. Biol. Chem 2001, 276, 36493–36500. [Google Scholar]
- Dittrich, M.; Freddolino, P.L.; Schulten, K. When light falls in LOV: A quantum mechanical/molecular mechanics study of photoexcitation in Phot-LOV1 of Chlamydomonas reinhardtii. J. Phys. Chem. B 2005, 109, 13006–13013. [Google Scholar]
- Gauden, M.; Stokkum, I.H.M.; van Key, J.M.; Luhrs, D.C.; Grondelle, R.; van Hegemann, P.; Kennis, J.T.M. Hydrogen-bond switching through a radical pair mechanism in a flavin-binding photoreceptor. Proc. Natl. Acad. Sci. USA 2006, 103, 10895–10900. [Google Scholar]
- Bonetti, C.; Mathes, T.; Stokkum, I.H.M.; van Mullen, K.M.; Groot, M.-L.; Grondelle, R.; van Hegemann, P.; Kennis, J.T.M. Hydrogen bond switching among flavin and amino acid side chains in the BLUF photoreceptor observed by ultrafast infrared spectroscopy. Biophys. J 2008, 95, 4790–4802. [Google Scholar]
- Bonetti, C.; Stierl, M.; Mathes, T.; Stokkum, I.H.M.; van Mullen, K.M.; Cohen-Stuart, T.A.; Grondelle, R.; Hegemann, P.; van Kennis, J.T.M. The role of key amino acids in the photoactivation pathway of the Synechocystis Slr1694 BLUF domain. Biochemistry 2009, 48, 11458–11469. [Google Scholar]
- Mathes, T.; Stokkum, I.H.M.; van Bonetti, C.; Hegemann, P.; Kennis, J.T.M. The hydrogen-bond switch reaction of the Blrb Bluf Domain of Rhodobacter sphaeroides. J. Phys. Chem. B 2011, 115, 7963–7971. [Google Scholar]
- Green, B.R. The Evolution of Light-Harvesting Antennas. In Light-Harvesting Antennas in Photosynthesis (Advances in Photosynthesis and Respiration); Green, B.R., Parson, W.W., Eds.; Kluwer Academic Publishers (Springer Link): Dordrecht, The Netherlands, 2003; Volume 13, pp. 129–168. [Google Scholar]
- Jordan, P.; Fromme, P.; Witt, H.T.; Klukas, O.; Saenger, W.; Krauβ, N. Three-dimensional structure of cyanobacterial photosystem I at 2.5Å resolution. Nature 2001, 411, 909–916. [Google Scholar]
- Dawson, M.C.; Elliott, D.C.; Elliott, W.H.; Jones, K.M. Data for Biochemical Research, 2nd ed; Clarendon Press: Oxford, UK, 1986; p. 580. [Google Scholar]
- Brown, S.B.; Holroyd, J.A.; Troxler, R.F.; Offenert, G.D. Bile pigment synthesis in plants. Incorporation of haem into phycocyanobilin and phycobiliproteins in Cyanidium caldarium. Biochem. J 1981, 194, 137–147. [Google Scholar]
- Zechmeister, L. cis-trans Isomeric Carotenoids Vitamins A and Arylpolyenes; Springer-Verlag: Vienna, Austria, 1962. [Google Scholar]
- Lucock, M.D. Folic acid: Nutritional biochemistry, molecular biology, and role in disease processes (minireview). Mol. Genet. MeTable 2000, 71, 121–138. [Google Scholar]
- Eirich, L.D.; Vogels, G.D.; Wolfe, R.S. Proposed structure for coenzyme F420 from Methanobacterium. Biochemistry 1978, 17, 4583–4593. [Google Scholar]
- Rabinowitz, J.C. Preparation and properties of 5,10-methenyltetrahydrofolic acid and 5-formyltetrahydrofolic acid. Methods Enzymol 1963, 6, 814–815. [Google Scholar]
- Bair, T.B.; Isabelle, D.W.; Daniels, L. Structures of coenzyme F420 in Mycobacterium species. Arch. Microbiol 2001, 176, 37–43. [Google Scholar]
- Klar, T.; Kaiser, G.; Hennecke, U.; Carell, T.; Batschauer, A.; Essen, L-O. Natural and non-natural antenna chromophores in the DNA photolyase from Thermus thermophilus. Chem Bio Chem 2006, 7, 1798–1806. [Google Scholar]
- Fujihashi, M.; Numoto, N.; Kobayashi, Y.; Mizushima, A.; Tsujimura, M.; Nakamura, A.; Kawarabayasi, Y.; Miki, K. Crystal structure of archaeal photolyase from Sulfolobus tokodaii with two FAD molecules: Implication of a novel light-harvesting cofactor. J. Mol. Biol 2007, 365, 903–910. [Google Scholar]
- Geisselbrecht, Y.; Frühwirth, S.; Schroeder, C.; Pierik, A.J.; Klug, G.; Essen, L.-O. CryB from Rhodobacter sphaeroides: A unique class of cryptochromes with new cofactors. EMBO Reports 2012, 13, 223–229. [Google Scholar]
- Telegina, T.A.; Lyudnikova, T.A.; Zemskova, Y.L.; Kritsky, M.S. Resistance of 5,10-methenyltetrahydrofolate to ultraviolet radiation. Appl. Biochem. Microbiol 2005, 41, 275–282. [Google Scholar]
- Schmidt, W. Bluelight-induced, flavin-mediated transport of redox equivalents across artificial bilayer membranes. J. Membr. Biol 1984, 82, 113–122. [Google Scholar]
- Kaler, J.B. Stars and Their Spectra: An Introduction to the Spectral Sequence; Cambridge University Press: Cambridge, UK, 1997; p. 300. [Google Scholar]
- Ahmed, T. The Wavelength of the Sun’s Peak Radiation Output. The Physics Factbook—An Encyclopedia of Scientific Essays. Elert, G., Ed.; 2002. Available online: http://hypertextbook.com/facts/2002/TahirAhmed.shtml accessed on 24 December 2012.
- Holland, H.D. The oxygenation of the atmosphere and oceans. Phil. Trans. Royal Soc 2006, 361, 903–915. [Google Scholar]
- Müller, F.; Brüstlein, M.; Hemmerich, P.; Massey, V.; Walker, W.H. Light-absorption studies on neutral flavin radicals. Eur. J. Biochem 1972, 25, 573–580. [Google Scholar]
- Ehrenberg, A.; Müller, F.; Hemmerich, P. Basicity, visible spectra, and electron spin resonance of flavosemiquinone anions. Eur. J. Biochem 1967, 2, 286–293. [Google Scholar]
- Su, Y.; Tripathi, G.N.R. Time-resolved resonance Raman observation of protein-free riboflavin semiquinone radicals. J. Am. Chem. Soc 1994, 116, 4405–4407. [Google Scholar]
- Land, E.J.; Swallow, A.J. One-electron reactions in biochemical systems as studied by pulse radiolysis. II. Riboflavin. Biochemistry 1969, 8, 2117–2125. [Google Scholar]
- Edwards, A.M. General Properties of Flavins. In Flavins: Photochemistry and Photobiology. Comprehensive Series in Photochemistry and Photobiology; Silva, E., Edwards, A.M., Eds.; Royal Society of Chemistry: London, UK, 2006; Volume 6, pp. 1–11. [Google Scholar]
- Penzkofer, A. Reduction-oxidation photocycle dynamics of flavins in starch films. Int. J. Mol. Sci 2012, 13, 9157–9183. [Google Scholar]
- Borda, M.J.; Elsetinow, A.R.; Schoonen, M.A.; Strongin, D.R. Pyrite-induced hydrogen peroxide formation as a driving force in the evolution of photosynthetic organisms on an early Earth. Astrobiology 2001, 1, 283–288. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 6th ed; W.H. Freeman: New York, NY, USA, 2007; p. 413. [Google Scholar]

| Parameter | Photosynthetic apparatus | Bacteriorhodopsin-driven mechanism | Flavin-based system | |
|---|---|---|---|---|
| Flavoprotein photoreceptors | Model of abiogenic photophosphorylation | |||
| Chromophore of the photochemically active pigment | Mg-porphyrin Chlorophyll or bacteriochlorophyll | Isoprenoid Retinal (all-trans and 13-cis) | Isoalloxazine Flavin (FMN, FAD) | Isoalloxazine, Pteridine |
| Antenna pigments | Mg-porphyrin (Chlorophylls or bacteriochlorophylls Polyene (carotenoids) Linear tetrapyrrole (bilins) | No | Deazaflavin, Pterin (MTHF), Isoalloxazine (FMN, FAD) | No data available |
| Active spectral range, nm | approx. 400/800 | approx. 500/650 | approx. 320/500 (For oxidized molecules) | approx. 320/500 (For oxidized molecules) |
| Microenvironment of the reaction center (photocatalytic center) and antenna | Lipid membrane | Lipid membrane | Protein molecule in aqueous medium | Matrix surrounded with aqueous medium |
| Involvement of excited pigment in energy transfer | Yes | No | Yes (in some photoreceptors) | No data available |
| Conversion of photon energy into the energy of ATP | Yes | Yes | No | Yes |
| NAP/NRCP* | ≈ 10/103 | No | = 1 | No data available |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kritsky, M.S.; Telegina, T.A.; Vechtomova, Y.L.; Buglak, A.A. Why Flavins Are not Competitors of Chlorophyll in the Evolution of Biological Converters of Solar Energy. Int. J. Mol. Sci. 2013, 14, 575-593. https://doi.org/10.3390/ijms14010575
Kritsky MS, Telegina TA, Vechtomova YL, Buglak AA. Why Flavins Are not Competitors of Chlorophyll in the Evolution of Biological Converters of Solar Energy. International Journal of Molecular Sciences. 2013; 14(1):575-593. https://doi.org/10.3390/ijms14010575
Chicago/Turabian StyleKritsky, Mikhail S., Taisiya A. Telegina, Yulia L. Vechtomova, and Andrey A. Buglak. 2013. "Why Flavins Are not Competitors of Chlorophyll in the Evolution of Biological Converters of Solar Energy" International Journal of Molecular Sciences 14, no. 1: 575-593. https://doi.org/10.3390/ijms14010575
APA StyleKritsky, M. S., Telegina, T. A., Vechtomova, Y. L., & Buglak, A. A. (2013). Why Flavins Are not Competitors of Chlorophyll in the Evolution of Biological Converters of Solar Energy. International Journal of Molecular Sciences, 14(1), 575-593. https://doi.org/10.3390/ijms14010575