Trends in Nanopharmaceutical Patents
Abstract
:1. Introduction
- ✓
- The new techniques involving molecular images, which allow us to understand the physiological processes of the human body on the nanoscale, thus leading to the development of methods to diagnose and treat diseases more accurately and sensitively. One example in particular is superparamagnetic nanoparticles using proteins for the molecular imaging of cancer;
- ✓
- The new qualitative tools of analysis that will allow for a better understanding of the working of cells at a molecular level and, particularly, the cells related to diseases. The union of proteomic and metabolomic genes into nanobiotechnology will allow for the development of more efficient and specific medicines for each type of disease. Another benefit will be the development of more sensitive and specific ex vivo tests;
- ✓
- Nanosensors and nanorobots, which when introduced into the body orally or intravenously, can identify and destroy cancerous cells or those infected by a virus, as well as regenerate damaged issue. These nanorobots can also be used to monitor vital functions (such as measuring arterial pressure or checking the level of glucose in the blood) in patients that require constant medical care.
Retrieving Patent Documents Related to Nanotechnology in Pharmaceutical Area
- ✓
- The possibility of exporting the patent documents recovered in the search, in their entirety, using software designed to work with this type of information, such as, for example, the commercial software, Vantage Point®[7], used in the treatment of the patent data included in this article;
- ✓
- The availability of Manual Codes, a classification attributed in the database to all the patent documents indexed in it. This classification also has a hierarchical structure, such as the International Classification of Patents (IPC) [8], although in less detail. The Manual Codes [9] divide the technological knowledge contained in the patent documents into 21 sections.
- ✓
- Toxicity of nanoparticle;
- ✓
- Nano-imaging agent;
- ✓
- Nanoparticle for drug delivery;
- ✓
- Cancer-targeting nanoparticle.
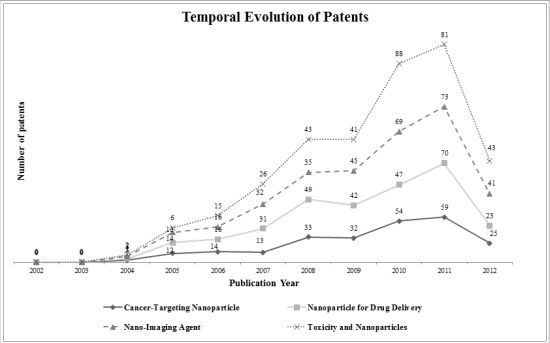
2. Results and Discussion
2.1. Toxicity and Nanoparticles
2.2. Nano-Imaging Agent
2.3. Nanoparticle for Drug Delivery
2.4. Cancer-Targeting Nanoparticle
3. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Seeking Alpha. DNA Nanotechnology: 3 Companies Building Better Synthetic Vaccines. Available online: http://seekingalpha.com/article/853551-dna-nanotechnology-3-companies-building-better-synthetic-vaccines (accessed on 11 December 2012).
- Sahoo, S.K.; Parveen, S.; Panda, J.J. The present and future of nanotechnology in human health care. Nanomedicine 2007, 3, 20–31. [Google Scholar]
- The Freedonia Group. Nanotechnology in Health Care: US Industry Study with Forecasts to 2011, 2016 & 2021. Available online: http://www.freedoniagroup.com/brochure/21xx/2168smwe.pdf (accessed on 7 December 2012).
- Alencar, M.S.M. Study of the Future through the Application of Technological Prospecting Techniques: The Case of Nanotechnology. Ph.D. Thesis, Federal University of Rio de Janeiro, November 2008. [Google Scholar]
- Schmidt, S.R. Fusion-proteins as biopharmaceuticals—Applications and challenges. Curr. Opin. Drug Discov. Devel 2009, 12, 284–295. [Google Scholar]
- Alencar, M.S.M.; Porter, A.L.; Antunes, A.M.S. Nanopatenting patterns in relation to product life cycle. Technol. Forecast. Soc. Change 2007, 74, 1661–1680. [Google Scholar]
- The Vantage Point. Privately owned software developed by the North American company; Search Technology, capable of importing and dealing with text and numeric data from data bases, and not just those related to patents. After establishing the required criteria, this software is capable of grouping the data obtained in pre-determined series, establishing correlations between them. Other benefits are the capacity to normalize data and generate reports with the results. Available online: http://www.thevantagepoint.com/ (accessed on 7 December 2012).
- International Patent Classification. The IPC was established in 1975 introduced after the Strasbourg Accord and is run by the World Intellectual Property Organization (WIPO). The aim of this system is to make patent documents available in an organized and standardized way, to make the technological and legal information contained in them easily accessible (using search tools). The IPC is currently used by more than 100 countries and the updated version is available at the WIPO site. Available online: http://www.wipo.int/classifications/ipc/ (accessed on 7 December 2012).
- Manual Code. Available online: http://ip-science.thomsonreuters.com/support/patents/dwpiref/reftools/classification/mc-manuals (accessed on 17 September 2012).
- Lucien, M.P.R.; Acar, H.; Bonitatatebus, P.J.J.; Dixon, W.T.; Kulkarni, A.M. Nanoparticles with inorganic core and methods of using them. U.S. Patent 2006/0018835, 26 January 2006. [Google Scholar]
- Bonitatibus, P.J.; Butts, M.D.; Colborn, R.E.; Kulkarni, A.M.; Hay, B.A.; Torres, A.S.; Bales, B.C.; Marino, M.E. Nanoparticle Contrast Agents for Diagnostic Imaging. U.S. Patent 2010/278748, 4 November 2010. [Google Scholar]
- Hay, B.A.; Bales, B.C.; Luttrell, M.T.; Kandapallil, B.I.I. Diagnostic Agent Composition and Associated Methods Thereof. U.S. Patent 2012/0156142, 21 June 2012. [Google Scholar]
- Bonitatibus, P.J.; Butts, M.D.; Colborn, R.E.; Kulkarni, A.M.; Hay, B.A.; Torres, A.S.; Bales, B.C.; Marino, M.E. Nanoparticle Contrast Agents for Diagnostic Imaging. U.S. Patent 2010/278749, 4 November 2010. [Google Scholar]
- Butts, M.D.; Colborn, R.E.; Bonitatibus, P.J.; Kulkarni, A.M.; Hay, B.A.; Torres, A.S.; Bales, B.C.; Marino, M.E. Nanoparticle Contrast Agents for Diagnostic Imaging. U.S. Patent 2010/166664, 7 July 2010. [Google Scholar]
- Butts, M.D.; Colborn, R.E.; Bonitatibus, P.J.; Kulkarni, A.M.; Hay, B.A.; Torres, A.S.; Bales, B.C.; Marino, M.E. Nanoparticle Contrast Agents for Diagnostic Imaging. U.S. Patent 2010/166665, 7 July 2010. [Google Scholar]
- Gu, F.X.; Teply, B.A.; Langer, R.S.; Farokhzad, O.C. Polymers for Functional Particles. WO 2007133807, 22 November 2007. [Google Scholar]
- Mirkin, C.A.; Giljohann, D.A.; Daniel, W.L.; Lippard, S.J.; Dhar, S. Polyvalent Polynucleotide Nanoparticle Conjugates as Delivery Vehicles for a Chemotherapeutic Agent WO/2011/028847, 10 March 2011.
- Cunningham, P.H.; Wood, K.G.; Langer, R.; Litlle, S. Hierarchically Self-assembling Linear-Dendritic Hybrid Polymers for Delivery of Biologically Active Agents. WO/2007/002663, 4 January 2007. [Google Scholar]
- Capila, I.; Sengupta, S.; Zhao, G.; Eavarone, D.; Sasisekharan, R. Nanocell Drug Delivery System WO/2008/014478, 31 January 2008.
- Nguyen, D.; Anderson, D.G.; Langer, R.; Mahon, K.P. Modulation of the Immune Response WO/2010/062322, 3 June 2010.
- Desai, N.P.; Soon-Shiong, P. Methods and Compositions for Treating Pulmonary Hypertension WO/2008/137148, 13 November 2008.
- Desai, N.P.; Soon-Shiong, P.; Trieu, V. Compositions and methods of delivery of pharmacological agents U.S. Patent 2010/196490, 5 August 2010.
- Trieu, V.; D’Cruz, O.; Desai, N.P. Combinations and Modes of Administration of Therapeutic Agents and Combination Therapy WO/2010/068925, 17 June 2010.
- Liversidge, E.; Wei, L. Stabilization of Chemical Compounds Using Nanoparticulate Formulations U.S. Patent 2007/224279, 27 September 2007.
- Ryde, N.P.; Snyder, P.; Liu, W.; Slifer, D. Reduction of Flake-like Aggregation in Nanoparticulate Active Agent Compositions WO/2010/138539, 2 December 2010.
- Layre, A.M.; Gref, R.; Couvreur, P.; Richard, J. Nanoparticules Polymeriques Composites. France Patent 2006. [Google Scholar]
- Nel, A.E. Assessing the Toxic Potential of Nanomaterials WO/2008/010843, 24 January 2008.
- Size-dependent Biological Effect of Nanoparticles WO/2009/002386, 31 December 2008.
- Acar, H.Y.; Syud, F.A.; Garaas, R.N.; Bonitatebus, J.P.J.; Kulkarni, A.M. Contrast Agents for Magnetic Resonance Imaging U.S. Patent 2005/260137, 24 November 2005.
- Hurd, R.E. Magnetic Resonance Imaging (MRI) Agents: Water Soluble Carbon-13 Enriched Fullerene and Carbon Nanotubes for Use with Dynamic Nuclear Polarization U.S. Patent 2007/025918, 1 February 2007.
- Bonitatibus, P.J.; Axelsson, O.H.E.; Kulkarni, A.M.; Bales, B.C.; Walter, D.J.; Torres, A.S.; Treynor, C. Nanoparticle-based Imaging Agents for X-ray/Computed Tomography U.S. Patent 2007/098640, 3 May 2007.
- Weiss, S.; Bruchez, M.J.; Alivisatos, P. Organo Luminescent Semiconductor Nanocrystal Probes for Biological Applications and Process for Making and Using Such Probes U.S. Patent 2006/003464, 5 January 2006.
- Chen, F.F.; Gerion, D.; Gray, J.W.; Budinger, T.F. Multimodal Imaging Probes for in VivoTargeted and Non-targeted Imaging and Therapeutics WO/2009/045579, 9 April 2009.
- Chen, F.F.; Bouchard, L. Multi-modality Nanoparticles Having Optically Responsive Shape WO/2010/096828, 26 August 2010.
- Van Bommel, T.; Willard, N.P. Ultrasound Contrast Agents for Molecular Imaging WO/2005/070473, 4 August 2005.
- Willard, N.P.; Van Bommel, T. Ultrasound Contrast Agents for Molecular Imaging WO/2006/054240, 26 May 2006.
- Gao, J.; Hillebrenner, H.L. Nanotubular Probes as Ultrasensitive MR Contrast Agent U.S. Patent 2008/124281, 29 May 2008.
- Yuan, Z.; Seo, S. Methods and Compositions for Nanoparticle-Mediated Cancer Cell-Targeted Delivery WO/2011/088456, 21 July 2011.
- Sung, H.; TU, H. Nanoparticles for Protein Drug Delivery. U.S. Patent 7863259, 4 January 2011. [Google Scholar]
- JIN, S.; OH, S. Articles Comprising Large-Surface-Area Bio-Compatible Materials and Methods for Making and Using Them WO/2008/066965, 5 June 2008.
- Zhang, L.; Aryal, S.; Hu, C. Ratiometric Combinatorial Drug Delivery WO/2011/143201, 17 November 2011.
- Zugates, G.T.; Zumbuehl, A.; Langer, R.S.; Anderson, D.G. End-Modified Poly(beta-amino esters) and Uses Thereof WO/2008/011561, 24 January 2008.
- Alexis, F.; Zhang, L.; Radovic-Moreno, A.F.; Gu, F.X.; Basto, P.; Levy-Nissenbaum, E.; Chan, J.; Langer, R.S.; Farokhzad, O.C. Poly(amino acid) Targeting Moieties WO/2008/124639, 16 October 2008.
- Von Andrian, U.H.; Farokhzad, O.C.; Langer, R.S.; Junt, T.; Moseman, A.; Zhang, L.; Basto, P.; Iannacone, M.; Alexis, F. Vaccine Nanotechnology WO/2009/051837, 23 April 2009.
- Von Maltzahn, G.A.; Bhatia, S.N. Methods and Systems for Treatment and/or Diagnosis WO/2010/101627, 10 Sepetember 2010.
- Farokhzad, O.C.; Salvador-Morales, C.; Gao, W.; Zhang, L.; Chen, J.M.; Langer, R.S. Particles with Multiple Functionalized Surface Domains WO/2010/042555, 15 April 2010.
- Lam, K.S.; Liu,, R; Peng,, L. Alpha-4 Beta-1 Integrin Ligands for Imaging and Therapy U.S. Patent 2006/019900, 26 January 2006.
- Guo, T. Nanoparticle Radiosensitizers WO/2006/037081, 6 April 2006.


| Derwent manual code | Description | Number of patent documents |
|---|---|---|
| B12-M11Q | Nanoparticles | 2,306 |
| B12-M10A7 | Nanotechnology devices | 160 |
| B11-C12 | Nanotechnology (general) | 5,028 |
| B05-U05A | Nanotubes, nanorods or nanohorns | |
| B05-U05B | nanofilms | 100 |
| B05-U05C | Nanostructures, other than those covered by B05-U05A and B05-U05B | |
| B05-U05 | Other carbon containing 3-D structures | 44 |
| B05-U04 | Carbon plus heteroatom nanotubes | 40 |
| B05-U03 | Carbon-only nanotubes | 615 |
| Keywords | Number of patent documents |
|---|---|
| toxicity and nanoparticle | 341 |
| nano-imaging agent | 327 |
| nanoparticle for drug delivery | 294 |
| cancer-targeting nanoparticle | 245 |
| nanoparticle cell interaction | 63 |
| nanoparticle protein interactions | 52 |
| toxicity of nanomaterials | 17 |
| Total of patents | Entity applying for patents | Toxicity of nanoparticle | Nano-imaging agent | Nanoparticle for drug delivery | Cancer-targeting nanoparticle |
|---|---|---|---|---|---|
| 23 | Univ. of California | 7 | 9 | 7 | 9 |
| 21 | Massachusetts Inst. Technology | 8 | 5 | 7 | 11 |
| 20 | General Electric Co | 10 | 20 | 0 | 0 |
| 16 | Univ. Nat. Tsing Hua | 0 | 0 | 12 | 0 |
| 15 | GP Medical Inc. | 0 | 0 | 11 | 0 |
| 12 | Univ. Texas System | 0 | 6 | 5 | 6 |
| 8 | Univ. Northwestern | 0 | 0 | 0 | 6 |
| 8 | Elan Pharma Int. Ltd. | 7 | 0 | 0 | 0 |
| 8 | Konink Philips Electronics NV | 0 | 8 | 0 | 0 |
| 7 | Centre National de La Recherche Scientifique (CNRS) | 7 | 0 | 0 | 5 |
| 7 | Abraxis Bioscience LLC | 7 | 0 | 0 | 0 |
| 7 | Fuji Film Co Ltd. | 0 | 0 | 6 | 0 |
| 6 | Brigham & Women’s Hospital Inc. | 0 | 0 | 0 | 6 |
| 5 | Univ. Kyushu | 0 | 0 | 5 | 0 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Antunes, A.; Fierro, I.; Guerrante, R.; Mendes, F.; Alencar, M.S.d.M. Trends in Nanopharmaceutical Patents. Int. J. Mol. Sci. 2013, 14, 7016-7031. https://doi.org/10.3390/ijms14047016
Antunes A, Fierro I, Guerrante R, Mendes F, Alencar MSdM. Trends in Nanopharmaceutical Patents. International Journal of Molecular Sciences. 2013; 14(4):7016-7031. https://doi.org/10.3390/ijms14047016
Chicago/Turabian StyleAntunes, Adelaide, Iolanda Fierro, Rafaela Guerrante, Flavia Mendes, and Maria Simone de M. Alencar. 2013. "Trends in Nanopharmaceutical Patents" International Journal of Molecular Sciences 14, no. 4: 7016-7031. https://doi.org/10.3390/ijms14047016
APA StyleAntunes, A., Fierro, I., Guerrante, R., Mendes, F., & Alencar, M. S. d. M. (2013). Trends in Nanopharmaceutical Patents. International Journal of Molecular Sciences, 14(4), 7016-7031. https://doi.org/10.3390/ijms14047016