Frequency-Dependent Magnetic Susceptibility of Magnetite and Cobalt Ferrite Nanoparticles Embedded in PAA Hydrogel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Magnetic Nanoparticles
2.2. Surface Modification of Magnetite Nanoparticles Using Acrylic Acid
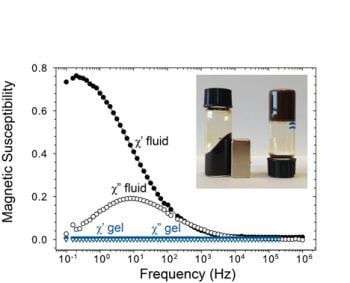
2.3. Magnetic Relaxation of Ferrogels
3. Experimental Section
3.1. Materials
3.2. Preparation of Magnetic Nanoparticles
3.3. Positive Charging of Magnetic Nanoparticles
3.4. Surface Modification of Magnetic Nanoparticles with Oleic Acid
3.5. Preparation of Hydrogel and Fluid Samples for Magnetic Susceptibility Measurements
3.6. Characterization
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar]
- Qin, J.; Asempah, I.; Laurent, S.; Fornara, A.; Muller, R.N.; Muhammed, M. Injectable superparamagnetic ferrogels for controlled release of hydrophobic drugs. Adv. Mater 2009, 21, 1354–1357. [Google Scholar]
- Pankhurst, Q.; Connolly, J.; Jones, S.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys 2003, 36, R167–R181. [Google Scholar]
- Han, I.; Han, M.; Kim, J.; Lew, S.; Lee, Y.; Horkay, F.; Magda, J. Constant-volume hydrogel osmometer: A new device concept for miniature biosensors. Biomacromolecules 2002, 3, 1271–1275. [Google Scholar]
- Urban, G.A.; Weiss, T. Hydrogels for Biosensors. In Hydrogel Sensors and Actuators: Engineering and Technology, 2010 ed; Gerlach, G., Arndt, K.-F., Eds.; Springer: Berlin, Germany, 2009; pp. 197–220. [Google Scholar]
- Van der Linden, H.; Herber, S.; Olthuis, W.; Bergveld, P. Stimulus-sensitive hydrogels and their applications in chemical (micro)analysis. Analyst 2003, 128, 325–331. [Google Scholar]
- Van Bruggen, M.P.B.; van Zon, J.B.A. Theoretical description of a responsive magneto-hydrogel transduction principle. Sens. Actuators A Phys 2010, 158, 240–248. [Google Scholar]
- Ilg, P. Stimuli-responsive hydrogels cross-linked by magnetic nanoparticles. Soft Matter 2013, 9, 3465–3468. [Google Scholar]
- Messing, R.; Frickel, N.; Belkoura, L.; Strey, R.; Rahn, H.; Odenbach, S.; Schmidt, A.M. Cobalt ferrite nanoparticles as multifunctional cross-linkers in PAAm ferrohydrogels. Macromolecules 2011, 44, 2990–2999. [Google Scholar]
- Vo, D.Q.; Kim, E.; Kim, S. Surface modification of hydrophobic nanocrystals using short-chain carboxylic acids. J. Colloid Interface Sci 2009, 337, 75–80. [Google Scholar]
- Astalan, A.; Ahrentorp, F.; Johansson, C.; Larsson, K.; Krozer, A. Biomolecular reactions studied using changes in Brownian rotation dynamics of magnetic particles. Biosens. Bioelectron 2004, 19, 945–951. [Google Scholar]
- Chung, S.; Hoffmann, A.; Bader, S.; Liu, C.; Kay, B.; Makowski, L.; Chen, L. Biological sensors based on Brownian relaxation of magnetic nanoparticles. Appl. Phys. Lett 2004, 85, 2971–2973. [Google Scholar]
- Kotitz, R.; Weitschies, W.; Trahms, L.; Brewer, W.; Semmler, W. Determination of the binding reaction between avidin and biotin by relaxation measurements of magnetic nanoparticles. J. Magn. Magn. Mater 1999, 194, 62–68. [Google Scholar]
- Fornara, A.; Johansson, P.; Petersson, K.; Gustafsson, S.; Qin, J.; Olsson, E.; Ilver, D.; Krozer, A.; Muhammed, M.; Johansson, C. Tailored magnetic nanoparticles for direct and sensitive detection of biomolecules in biological samples. Nano Lett 2008, 8, 3423–3428. [Google Scholar]
- Hong, C.; Wu, C.; Chiu, Y.; Yang, S.; Horng, H.; Yang, H. Magnetic susceptibility reduction method for magnetically labeled immunoassay. Appl. Phys. Lett 2006, 88, 212512, :1–212512:3.. [Google Scholar]
- Barrera, C.; Florian-Algarin, V.; Acevedo, A.; Rinaldi, C. Monitoring gelation using magnetic nanoparticles. Soft Matter 2010, 6, 3662–3668. [Google Scholar]
- Erne, B.H.; Butter, K.; Kuipers, B.W.M.; Vroege, G.J. Rotational diffusion in iron ferrofluids. Langmuir 2003, 19, 8218–8225. [Google Scholar]
- Fannin, P. Wideband measurement and analysis techniques for the determination of the frequency-dependent, complex susceptibility of magnetic fluids. Adv. Chem. Phys 1998, 104, 181–292. [Google Scholar]
- Rosensweig, R. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater 2002, 252, 370–374. [Google Scholar]
- Chikazumi, S.; Charap, S.H. Physics of Magnetism, 1st ed; John Wiley & Sons, Inc: New York, NY, USA, 1964; p. 554. [Google Scholar]
- Luigjes, B.; Woudenberg, S.M.C.; de Groot, R.; Meeldijk, J.D.; Galvis, H.M.T.; de Jong, K.P.; Philipse, A.P.; Erne, B.H. Diverging geometric and magnetic size distributions of iron oxide nanocrystals. J. Phys. Chem. C 2011, 115, 14598–14605. [Google Scholar]
- Kodama, R. Magnetic nanoparticles. J. Magn. Magn. Mater 1999, 200, 359–372. [Google Scholar]
- Batlle, X.; Labarta, A. Finite-size effects in fine particles: Magnetic and transport properties. J. Phys. D Appl. Phys 2002, 35, R15–R42. [Google Scholar]
- Barker, A.; Cage, B.; Russek, S.; Stoldt, C. Ripening during magnetite nanoparticle synthesis: Resulting interfacial defects and magnetic properties. J. Appl. Phys 2005, 98, 063528, :1–063528:7.. [Google Scholar]
- Goya, G.; Berquo, T.; Fonseca, F.; Morales, M. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys 2003, 94, 3520–3528. [Google Scholar]
- Roca, A.G.; Morales, M.P.; O’Grady, K.; Serna, C.J. Structural and magnetic properties of uniform magnetite nanoparticles prepared by high temperature decomposition of organic precursors. Nanotechnology 2006, 17, 2783–2788. [Google Scholar]
- Tartaj, P.; Morales, M.D.; Veintemillas-Verdaguer, S.; Gonzalez-Carreno, T.; Serna, C.J. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys 2003, 36, R182–R197. [Google Scholar]
- Zhao, L.; Zhang, H.; Xing, Y.; Song, S.; Yu, S.; Shi, W.; Guo, X.; Yang, J.; Lei, Y.; Cao, F. Studies on the magnetism of cobalt ferrite nanocrystals synthesized by hydrothermal method. J. Solid State Chem 2008, 181, 245–252. [Google Scholar]
- Tang, C.; Wang, C.; Chien, S. Characterization of cobalt oxides studied by FT-IR, Raman, TPR and TG-MS. Thermochimica Acta 2008, 473, 68–73. [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides; VCH Verlagsgesellschaft: Weinheim, Germany, 1996; p. 573. [Google Scholar]
- Max, J.; Chapados, C. Infrared spectroscopy of aqueous carboxylic acids: Comparison between different acids and their salts. J. Phys. Chem. A 2004, 108, 3324–3337. [Google Scholar]
- Klokkenburg, M.; Hilhorst, J.; Erne, B.H. Surface analysis of magnetite nanoparticles in cyclohexane solutions of oleic acid and oleylamine. Vib. Spectrosc 2007, 43, 243–248. [Google Scholar]
- Pretsch, E.; Buhlmann, P.; Badertscher, M. Structure Determination of Organic Compounds; Springer-Verlag: Berlin, Germany, 2009. [Google Scholar]
- Klokkenburg, M.; Dullens, R.; Kegel, W.; Erne, B.; Philipse, A. Quantitative real-space analysis of self-assembled structures of magnetic dipolar colloids. Phys. Rev. Lett 2006, 96, 037203, :1–037203:4.. [Google Scholar]
- Klokkenburg, M.; Erne, B.H.; Wiedenmann, A.; Petukhov, A.V.; Philipse, A.P. Dipolar structures in magnetite ferrofluids studied with small-angle neutron scattering with and without applied magnetic field. Phys. Rev. E 2007, 75, 051408. [Google Scholar]
- Klokkenburg, M.; Erne, B.H. Comparison of reversible and irreversible dipolar assemblies in a ferrofluid. J. Magn. Magn. Mater 2006, 306, 85–91. [Google Scholar]
- Klokkenburg, M.; Erne, B.H.; Philipse, A.P. Thermal motion of magnetic iron nanoparticles in a frozen solvent. Langmuir 2005, 21, 1187–1191. [Google Scholar]
- Galicia, J.A.; Cousin, F.; Dubois, E.; Sandre, O.; Cabuil, V.; Perzynski, R. Static and dynamic structural probing of swollen polyacrylamide ferrogels. Soft Matter 2009, 5, 2614–2624. [Google Scholar]
- Galicia, J.A.; Cousin, F.; Dubois, E.; Sandre, O.; Cabuil, V.; Perzynski, R. Local structure of polymeric ferrogels. J. Magn. Magn. Mater 2011, 323, 1211–1215. [Google Scholar]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn 1981, 17, 1247–1248. [Google Scholar]
- Andres Verges, M.; Costo, R.; Roca, A.G.; Marco, J.F.; Goya, G.F.; Serna, C.J.; Morales, M.P. Uniform and water stable magnetite nanoparticles with diameters around the monodomain-multidomain limit. J. Phys. D Appl. Phys 2008, 41, 134003. [Google Scholar]
- Tourinho, F.; Franck, R.; Massart, R. Aqueous ferrofluids based on manganese and cobalt ferrites. J. Mater. Sci 1990, 25, 3249–3254. [Google Scholar]
- Kuipers, B.W.M.; Bakelaar, I.A.; Klokkenburg, M.; Erne, B.H. Complex magnetic susceptibility setup for spectroscopy in the extremely low-frequency range. Rev. Sci. Instrum 2008, 79, 013901. [Google Scholar]
- Erne, B.H.; Claesson, M.; Sacanna, S.; Klokkenburg, M.; Bakelaar, E.; Kuipers, B.W.M. Low-Frequency complex magnetic susceptibility of magnetic composite microspheres in colloidal dispersion. J. Magn. Magn. Mater 2007, 311, 145–149. [Google Scholar]









| MS (emu/g) | MR/MS | HC (kA/m) | λ | |
|---|---|---|---|---|
| Magnetite (S) | 54.8 (1.2) | 0.007 (0.001) | - | 0.4 |
| Magnetite (L) | 67.5 (0.3) | 0.187 (0.003) | 5.09 (0.05) | 10 |
| Cobalt ferrite | 38 (4) | 0.343 (0.013) | 34 (4) | 3 |
| MS (emu per g gel) | MR/MS | HC (kA/m) | |
|---|---|---|---|
| Magnetite (S) | 1.20 (0.06) | 0.009 (0.000) | - |
| Magnetite (L) | 1.63 (0.04) | 0.238 (0.006) | 9.91 (0.15) |
| Cobalt ferrite | 0.95 (0.10) | 0.375 (0.012) | 26.8 (0.4) |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Van Berkum, S.; Dee, J.T.; Philipse, A.P.; Erné, B.H. Frequency-Dependent Magnetic Susceptibility of Magnetite and Cobalt Ferrite Nanoparticles Embedded in PAA Hydrogel. Int. J. Mol. Sci. 2013, 14, 10162-10177. https://doi.org/10.3390/ijms140510162
Van Berkum S, Dee JT, Philipse AP, Erné BH. Frequency-Dependent Magnetic Susceptibility of Magnetite and Cobalt Ferrite Nanoparticles Embedded in PAA Hydrogel. International Journal of Molecular Sciences. 2013; 14(5):10162-10177. https://doi.org/10.3390/ijms140510162
Chicago/Turabian StyleVan Berkum, Susanne, Joris T. Dee, Albert P. Philipse, and Ben H. Erné. 2013. "Frequency-Dependent Magnetic Susceptibility of Magnetite and Cobalt Ferrite Nanoparticles Embedded in PAA Hydrogel" International Journal of Molecular Sciences 14, no. 5: 10162-10177. https://doi.org/10.3390/ijms140510162
APA StyleVan Berkum, S., Dee, J. T., Philipse, A. P., & Erné, B. H. (2013). Frequency-Dependent Magnetic Susceptibility of Magnetite and Cobalt Ferrite Nanoparticles Embedded in PAA Hydrogel. International Journal of Molecular Sciences, 14(5), 10162-10177. https://doi.org/10.3390/ijms140510162