Molecular Interaction of a New Antibacterial Polymer with a Supported Lipid Bilayer Measured by an in situ Label-Free Optical Technique
Abstract
:1. Introduction
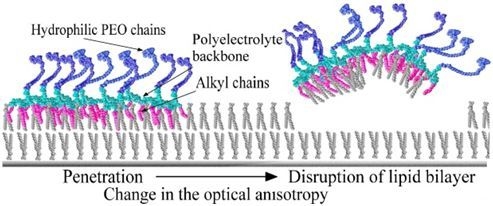
2. Results and Discussion
2.1. Isotropic Adlayer Model
2.2. Anisotropic Adlayer Model
2.3. Composite Model
3. Experimental Section
3.1. Materials
3.2. OWLS Measurement
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Ambroggio, E.E.; Bagatolli, L.A.; Goormaghtigh, E.; Fominaya, J.; Gasset, M. Piercing Lipid Bilayers with Peptides. In Advances in Planar Lipid Bilayers and Liposomes; Leitmannova Liu, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 5, pp. 1–23. [Google Scholar]
- Munoz-Bonilla, A.; Fernandez-Garcia, M. Polymeric materials with antimicrobial activity. Progr. Polym. Sci 2012, 37, 281–339. [Google Scholar]
- Papareddy, P.; Mörgelin, M.; Walse, B.; Schmidtchen, A.; Malmsten, M.J. Antimicrobial activity of peptides derived from human β-amyloid precursor protein. Pept. Sci 2012, 18, 183–191. [Google Scholar]
- Pelegrini, P.B.; Perseghini del Sarto, R.; Silva, O.N.; Franco, O.L. Grossi-de-Sa1, M.F. Antibacterial peptides from plants: What they are and how they probably work. Biochem. Res 2011. [Google Scholar] [CrossRef]
- Rossetti, F.F.; Reviakine, I.; Csucs, G.; Assi, F.; Voros, J.; Textor, M. Interaction of poly(l-lysine)-g-poly(ethylene glycol) with supported phospholipid bilayers. Biophys. J 2004, 87, 1711–1721. [Google Scholar]
- Zdyrko, B.; Klep, V.; Li, X.; Kang, Q.; Minko, S.; Wen, X.; Luzinov, I. Polymer brushes as active nanolayers for tunable bacteria adhesion. Mat. Sci. Eng. C Mater. Biol. Appl 2009, 29, 680–684. [Google Scholar]
- Ang, P.K.; Loh, K.P.; Wohland, T.; Nesladek, M.; Van Hove, E. Supported lipid bilayer on nanocrystalline diamond: Dual optical and field-effect sensor for membrane disruption. Adv. Funct. Mater 2009, 19, 109–116. [Google Scholar]
- Kuhn, P.; Eyer, K.; Allner, S.; Lombardi, D.; Dittrich, P.S. A microfluidic vesicle screening platform: Monitoring the lipid membrane permeability of tetracyclines. Anal. Chem 2011, 83, 8877–8885. [Google Scholar]
- Leung, S.J.; Kachur, X.M.; Bobnick, M.C.; Romanowski, M. Wavelength-selective light-induced release from plasmon resonant liposomes. Adv. Funct. Mater 2011, 21, 1113–1121. [Google Scholar]
- Mashaghi, A.; Swann, M.; Popplewell, J.; Textor, M.; Reimhult, E. Optical anisotropy of supported lipid structures probed by waveguide spectroscopy and its application to study of supported lipid bilayer formation kinetics. Anal. Chem 2008, 80, 3666–3676. [Google Scholar]
- Roiter, Y.; Ornatska, M.; Rammohan, A.R.; Balakrishnan, J.; Heine, D.R.; Minko, S. Interaction of nanoparticles with lipid membrane. Nano Lett 2008, 8, 941–944. [Google Scholar]
- Rossetti, F.F.; Textor, M.; Reviakine, I. Asymmetric distribution of phosphatidyl serine in supported phospholipid bilayers on titanium dioxide. Langmuir 2006, 22, 3467–3473. [Google Scholar]
- Brogden, K. Antimicrobial peptides: Pore formers of metabolic inhibitors in bacteria? Nat. Rev. Microbiol 2005, 3, 238–250. [Google Scholar]
- Chen, X.; Chen, Z. SFG Studies on Interactions between antimicrobial peptides and supported lipid bilayers. Biochim. Biophys. Acta 2006, 1758, 1257–1273. [Google Scholar]
- Helander, I.M.; Alakomi, H.; Latva-Kala, K.; Koski, P. Polyethyleneimine is an effective permeabilizer of gram-negative bacteria. Microbiology 1997, 143, 3193–3199. [Google Scholar]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1999, 1462, 55–70. [Google Scholar]
- Kenawy, E-R.; Worley, S.D.; Broughton, R. The Chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar]
- Lin, J.; Shuyi, Q.; Lewis, K.; Klibanov, A.M. Bactericidal properties of flat surfaces and nanoparticles derivatized with alkylated polyethylenimines. Biotechnol. Progr 2002, 18, 1082–1086. [Google Scholar]
- Lin, J.; Shuyi, Q.; Lewis, K.; Klibanov, A.M. On the mechanism of bactericidal and fungicidal activities of textiles covalently modified with alkylated polyethylenimine. Biotechnol. Bioeng 2003, 83, 168–172. [Google Scholar]
- Pasquier, N.; Keul, H.; Heine, E.; Moeller, M. From multifunctionalized poly(ethylene imine)s toward antimicrobial coatings. Biomacromolecules 2007, 8, 2874–2882. [Google Scholar]
- Werner, C. Polymers to permeate lipid bilayer membranes. Express Polym. Lett 2011, 5, 753. [Google Scholar]
- Fan, L.; Cao, M.; Gao, S.; Wang, W.; Peng, K.; Tan, C.; Wen, F.; Tao, S.; Xie, W. Preparation and characterization of a quaternary ammonium derivative of pectin. Carbohydr. Polym 2012, 88, 707–712. [Google Scholar]
- Wang, J.; Hu, W.; Liu, Q.; Zhang, S. Dual-functional composite with anticoagulant and antibacterial properties based on heparinized silk fibroin and chitosan. Colloids Surf. B 2011, 85, 241–247. [Google Scholar]
- Adelmann, R.; Mennicken, M.; Popescu, D.; Heine, E.; Keul, H.; Moeller, M. Functional polymethacrylates as bacteriostatic polymers. Eur. Polym. J 2009, 45, 3093–3107. [Google Scholar]
- Kenawy, E.-R.; Mahmoud, Y.A.-G. Biologically active polymers 6. Macromol. Biosci 2003, 3, 107–116. [Google Scholar]
- Waschinski, C.J.; Herdes, V.; Schueler, F.; Tiller, J.C. Influence of satellite groups on telechelic antimicrobial functions of polyoxazolines. Macromol. Biosci 2005, 5, 149–156. [Google Scholar]
- Kiss, É.; Heine, E.T.; Hill, K.; He, Y.-C.; Keusgen, N.; Pénzes, C.B.; Schnöller, D.; Gyulai, G.; Mendrek, A.; Keul, H.; et al. Membrane affinity and antibacterial properties of cationic polyelectrolytes with different hydrophobicity. Macromol. Biosci 2012, 12, 1181–1189. [Google Scholar]
- Pasquier, N.; Keul, H.; Heine, E.; Moeller, M.; Angelov, B.; Linser, S.; Willumeit, R. Amphiphilic branched polymers as antimicrobial agents. Macromol. Biosci 2008, 10, 903–915. [Google Scholar]
- Chen, X.; Tang, H.; Even, M.A.; Wang, J.; Tew, G.N.; Chen, Z.J. Observing a molecular knife at work. Am. Chem. Soc 2006, 128, 2711–2714. [Google Scholar]
- El Kirat, K.; Morandat, S.; Dufrêne, Y.F. Nanoscale analysis of supported lipid bilayers using atomic force microscopy. Biochim. Biophys. Acta 2010, 1798, 750–765. [Google Scholar]
- Shaw, J.E.; Alattia, J.-R.; Verity, J.E.; Privé, G.G.; Yip, C.M.J. Nanoscale analysis of supported lipid bilayers using atomic force microscopy. Struct. Biol 2006, 154, 42–58. [Google Scholar]
- Huang, W.; Zhang, Z.; Han, X.; Wang, J.; Tang, J.; Dong, S.; Wang, E. Concentration-dependent behavior of nisin interaction with supported bilayer lipid membrane. Biophys. Chem 2002, 99, 271–279. [Google Scholar]
- Chekmenev, E.Y.; Jones, S.M.; Nikolayeva, Y.N.; Vollmar, B.S.; Wagner, T.J.; Gorkov, P.L.; Brey, W.W.; Manion, M.N.; Daugherty, K.C.; Cotten, M.J. High-field NMR studies of molecular recognition and structure—Function relationships in antimicrobial piscidins at the water—Lipid bilayer interface. Am. Chem. Soc 2006, 128, 5308–5309. [Google Scholar]
- Rozek, A.; Friedrich, C.L. Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles. Biochemica 2000, 39, 15765–15774. [Google Scholar]
- Ha, T.H.; Kim, C.H. Interaction of indolicidin with model lipid bilayer: Quartz crystal microbalance and atomic force microscopy study. Langmuir 2000, 16, 871–875. [Google Scholar]
- Morandi, S.; Puggelli, M.; Caminati, G. Antibiotic association with phospholipid nano-assemblies: A comparison between Langmuir–Blodgett films and supported lipid bilayers. Colloids Surf A 2008, 321, 125–130. [Google Scholar]
- Mozsolits, H.; Wirth, H.-J.; Werkmeister, J.; Aguilar, M.I. Surface plasmon resonance spectroscopy: An emerging tool for the study of peptide–membrane interactions. Biochim. Biophys. Acta 2001, 1512, 64–76. [Google Scholar]
- Zhao, H.; Mattila, J.P. Comparison of the membrane association of two antimicrobial peptides, magainin 2 and indolicidin. Biophys. J 2001, 81, 2979–2991. [Google Scholar]
- Bahng, M.K.; Cho, N.J. Interaction of indolicidin with model lipid bilayers: FTIR-ATR spectroscopic study. Langmuir 1998, 14, 463–70. [Google Scholar]
- Hirst, D.J.; Lee, T-H.; Swann, M.J.; Unabia, S.; Park, Y.; Hahm, K.S.; Aguilar, M.I. Effect of acyl chain structure and bilayer phase state on binding and penetration of a supported lipid bilayer by HPA3. Eur. Biophys. J 2011, 40, 503–514. [Google Scholar]
- Lee, T.-H.; Heng, C.; Swann, M.J.; Gehman, J.D.; Separovic, F.; Aguilar, M.I. Real-time quantitative analysis of lipid disordering by aurein 1.2 during membrane adsorption, destabilisation and lysis. Biochim. Biophys. Acta 2010, 1798, 1977–1986. [Google Scholar]
- Alves, I.D.; Salgado, G.F.J.; Salamon, Z.; Brown, M.F.; Tollin, G.; Hruby, V.J. Phosphatidylethanolamine enhances rhodopsin photoactivation and transducin binding in a solid supported lipid bilayer as determined using plasmon-waveguide resonance spectroscopy. Biophys. J 2005, 88, 198–210. [Google Scholar]
- Salamon, Z.; Tollin, G. Optical anisotropy in lipid bilayer membranes: Coupled plasmon-waveguide resonance measurements of molecular orientation, polarizability, and shape. Biophys. J 2001, 80, 1557–1567. [Google Scholar]
- Csúcs, G.; Ramsden, J.J. Interaction of phospholipid vesicles with smooth metal-oxide surfaces. Biochim. Biophys. Acta Biomembr 1998, 1369, 61–70. [Google Scholar]
- Csúcs, G.; Ramsden, J.J. Solubilization of planar bilayers with detergent. Biochim. Biophys. Acta Biomembr 1998, 1369, 304–308. [Google Scholar]
- Horvath, R.; Fricsovszky, G.; Papp, E. Application of the optical waveguide lightmode spectroscopy to monitor lipid bilayer phase transition. Biosensors Bioelectron 2003, 18, 415–428. [Google Scholar]
- Michielin, O.; Ramsden, J.J.; Vergeres, G. Unmyristoylated MARCKS-related protein(MRP) binds to supported planar phosphatidylcholine membranes. Biochim. Biophys. Acta 1998, 1375, 110–116. [Google Scholar]
- Ramsden, J.J. Partial molar volume of solutes in bilayer lipid membranes. Phys. Chem 1993, 97, 4479–4483. [Google Scholar]
- Ramsden, J.J. Molecular orientation in lipid bilayers. Phil. Mag. B 1999, 79, 381–386. [Google Scholar]
- Sugihara, K.; Delai, M.; Szendrő, I.; Guillaume-Genti, O.; Vörös, J.; Zambelli, T. Simultaneous OWLS and EIS monitoring of supported lipid bilayers with the pore forming peptide melittin. Sensors Actuator B 2012, 161, 600–606. [Google Scholar]
- Vörös, J.; Ramsden, J.J.; Csúcs, G.; Szendrő, I.; de Paul, S.M.; Textor, M.; Spencer, N.D. Optical grating coupler biosensors. Biomaterials 2002, 23, 3699–3710. [Google Scholar]
- Aggarwal, N.; Lawson, K.; Kershaw, M.; Horvath, R.; Ramsden, J.J. Protein adsorption on heterogeneous surfaces. Appl. Phys. Lett 2009, 94, 083110. [Google Scholar] [Green Version]
- Du, Y.Z.; Saavedra, S.S. Molecular orientation distributions in protein films. v. Cytochrome cadsorbed to a sulfonate-terminated, self-assembled monolayer. Langmuir 2003, 19, 6443–6448. [Google Scholar]
- Fang, Y. Label-free cell-based assays with optical biosensors in drug discovery. Assay Drug Dev. Tech 2006, 4, 583–595. [Google Scholar]
- Kozma, P.; Hamori, A.; Kurunczi, S.; Cottier, K.; Horvath, R. Grating coupled optical waveguide interferometer for label-free biosensing. Sensors Actuator B 2011, 155, 446–450. [Google Scholar]
- Runge, A.F.; Mendes, S.B.; Saavedra, S.S.J. Order parameters and orientation distributions of solution adsorbed and microcontact printed cytochrome c protein films on glass and ITO. Phys. Chem. B 2006, 110, 6732–6739. [Google Scholar]
- Runge, A.F.; Rasmussen, N.C.; Saavedra, S.S. Determination of anisotropic optical constants and surface coverage of molecular films using polarized visible ATR spectroscopy. Application to adsorbed cytochrome c films. J. Phys. Chem. B 2005, 109, 424–431. [Google Scholar]
- Merz, Ch.; Knoll, W.; Textor, M.; Reimhult, E. Formation of supported bacterial lipid membrane mimics. Biointerphases 2008, 3, FA41–FA50. [Google Scholar]
- Seantier, B.; Kasemo, B. Influence of mono-and divalent ions on the formation of supported phospholipid bilayers via vesicle adsorption. Langmuir 2009, 25, 5767–5772. [Google Scholar]
- Ramsden, J.J. Review of new experimental techniques for investigating random sequential adsorption. Statist. Phys 1993, 73, 853–877. [Google Scholar]
- Tiefenthaler, K.; Lukosz, W.J. Sensitivity of grating couplers as integrated-optical chemical sensors. Opt. Soc. Am. B 1989, 6, 209–220. [Google Scholar]
- Ramsden, J.J. Proteins at Solid-Liquid Interfaces. In Principles and Practice; Déjardin, P., Ed.; Springer-Verlag: Heidelberg, Germany, 2006; pp. 23–49. [Google Scholar]
- Horvath, R.; Ramsden, J.J. Quasi-isotropic analysis of anisotropic thin films on optical waveguides. Langmuir 2007, 23, 9330–9334. [Google Scholar]
- De Feijter, J.A.; Benjamins, J.; Veer, F.A. Ellipsometry as a tool to study the adsorption behavior of synthetic and biopolymers at the air–water interface. Biopolymers 1978, 17, 1759–1772. [Google Scholar]
- Born, M.; Wolf, E. Principles of Optics, 7th ed; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Horvath, R.; McColl, J.; Yakubov, G.E.; Ramsden, J.J. Structural hysteresis and hierarchy in adsorbed glycoproteins. Chem. Phys 2008, 129, 071102. [Google Scholar]
- Cottier, K.; Horvath, R. Imageless microscopy of surface patterns using optical waveguides. Appl. Phys. B 2008, 91, 319–327. [Google Scholar]







© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Horvath, R.; Kobzi, B.; Keul, H.; Moeller, M.; Kiss, É. Molecular Interaction of a New Antibacterial Polymer with a Supported Lipid Bilayer Measured by an in situ Label-Free Optical Technique. Int. J. Mol. Sci. 2013, 14, 9722-9736. https://doi.org/10.3390/ijms14059722
Horvath R, Kobzi B, Keul H, Moeller M, Kiss É. Molecular Interaction of a New Antibacterial Polymer with a Supported Lipid Bilayer Measured by an in situ Label-Free Optical Technique. International Journal of Molecular Sciences. 2013; 14(5):9722-9736. https://doi.org/10.3390/ijms14059722
Chicago/Turabian StyleHorvath, Robert, Balázs Kobzi, Helmut Keul, Martin Moeller, and Éva Kiss. 2013. "Molecular Interaction of a New Antibacterial Polymer with a Supported Lipid Bilayer Measured by an in situ Label-Free Optical Technique" International Journal of Molecular Sciences 14, no. 5: 9722-9736. https://doi.org/10.3390/ijms14059722
APA StyleHorvath, R., Kobzi, B., Keul, H., Moeller, M., & Kiss, É. (2013). Molecular Interaction of a New Antibacterial Polymer with a Supported Lipid Bilayer Measured by an in situ Label-Free Optical Technique. International Journal of Molecular Sciences, 14(5), 9722-9736. https://doi.org/10.3390/ijms14059722