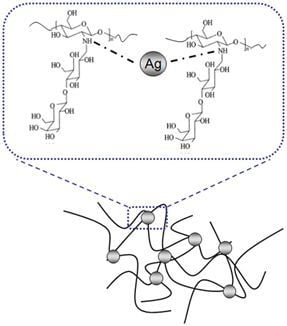
The Effect of a Silver Nanoparticle Polysaccharide System on Streptococcal and Saliva-Derived Biofilms
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.2. Discussion
3. Experimental Section
3.1. Bacterial Strains and Saliva Collection
3.2. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.3. Chitlac-nAg Effect on Biofilm Formation
3.4. Chitlac-nAg Effect on Mature Biofilm
3.5. MTT Assay of Metabolic Biofilm Activity
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kolenbrander, P.E. Multispecies communities: Interspecies interactions influence growth on saliva as sole nutritional source. Int. J. Oral Sci 2011, 3, 49–54. [Google Scholar]
- Lewis, K. Persister cells: Molecular mechanisms related to antibiotic tolerance. Handb. Exp. Pharmacol 2012, 211, 121–133. [Google Scholar]
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med 2012, 272, 541–561. [Google Scholar]
- Filoche, S.; Wong, L.; Sissons, C.H. Oral biofilms: Emerging concepts in microbial ecology. J. Dent. Res 2010, 89, 8–18. [Google Scholar]
- Napimoga, M.H.; Höfling, J.F.; Klein, M.I.; Kamiya, R.U.; Gonçalves, R.B. Tansmission, diversity and virulence factors of Streptococcus mutans genotypes. J. Oral Sci 2005, 47, 59–64. [Google Scholar]
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ 2005, 83, 661–669. [Google Scholar]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci 2012, 37, 281–339. [Google Scholar]
- Iannitelli, A.; Grande, R.; Di Stefano, A.; Di Giulio, M.; Sozio, P.; Bessa, L.J.; Laserra, S.; Paolini, C.; Protasi, F.; Cellini, L. Potential antibacterial activity of carvacrol-loaded poly(DL-lactide-co-glycolide) (PLGA) nanoparticles against microbial biofilm. Int. J. Mol. Sci 2011, 12, 5039–5051. [Google Scholar]
- Mahapatro, A.; Singh, D.K. Biodegradable nanoparticles are excellent vehicle for site directed in vivo delivery of drugs and vaccines. J. Nanobiotechnol 2011, 9, 55. [Google Scholar]
- Loh, X.J.; Lee, T.C. Gene delivery by functional inorganic nanocarriers. Recent Pat. DNA Gene Seq 2012, 6, 108–114. [Google Scholar]
- Gupta, A.S. Nanomedicine approaches in vascular disease: A review. Nanomedicine 2011, 7, 763–779. [Google Scholar]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed 2012, 7, 6003–6009. [Google Scholar]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol 2008, 74, 2171–2178. [Google Scholar]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2001, 27, 76–83. [Google Scholar]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Deimling, D.; Jaser, C.; Pelz, K.; Wittmer, A.; Ratka-Krüger, P. An antimicrobial effect from silver-coated toothbrush heads. Am. J. Dent 2010, 23, 251–254. [Google Scholar]
- Masurkar, S.A.; Chaudhari, P.R.; Shidore, V.B.; Kamble, S.P. Effect of biologically synthesised silver nanoparticles on Staphylococcus aureus biofilm quenching and prevention of biofilm formation. IET Nanobiotechnol 2012, 6, 110–114. [Google Scholar]
- Cabal, B.; Cafini, F.; Esteban-Tejeda, L.; Alou, L.; Bartolomé, J.F.; Sevillano, D.; López-Piriz, R.; Torrecillas, R.; Moya, J.S. Inhibitory effect on in vitro Streptococcus oralis biofilm of a soda-lime glass containing silver nanoparticles coating on titanium alloy. PLoS One 2012, 7, e42393. [Google Scholar]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf. B 2010, 79, 340–344. [Google Scholar]
- Travan, A.; Pelillo, C.; Donati, I.; Marsich, E.; Benincasa, M.; Scarpa, T.; Semeraro, S.; Turco, G.; Gennaro, R.; Paoletti, S. Non-cytotoxic silver nanoparticle-polysaccharide nanocomposites with antimicrobial activity. Biomacromolecules 2009, 10, 1429–1435. [Google Scholar]
- Grunlan, J.C.; Choi, J.K.; Lin, A. Antimicrobial behavior of polyelectrolyte multilayer films containing cetrimide and silver. Biomacromolecules 2005, 6, 1149–1153. [Google Scholar]
- Kumar, M.N.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev 2004, 104, 6017–6084. [Google Scholar]
- Huang, H.; Yuan, Q.; Yang, X. Preparation and characterization of metal-chitosan nanocomposites. Colloids Surf. B 2004, 39, 31–37. [Google Scholar]
- Marsich, E.; Travan, A.; Donati, I.; Turco, G.; Kulkova, J.; Moritz, N.; Aro, H.T.; Crosera, M.; Paoletti, S. Biological responses of silver-coated thermosets: An in vitro and in vivo study. Acta Biomater 2013, 9, 5088–5099. [Google Scholar]
- Travan, A.; Marsich, E.; Donati, I.; Benincasa, M.; Giazzon, M.; Felisari, L.; Paoletti, S. Silver-polysaccharide nanocomposite antimicrobial coatings for methacrylic thermosets. Acta Biomater 2011, 7, 337–346. [Google Scholar]
- Damian, M.; Palade, A.M.; Băltoiu, M.; Petrini, A.; Păuna, M.; Roseanu, A. Phenotypic and molecular methods used for identification of oral streptococci and related microorganisms. Roum. Arch. Microbiol. Immunol 2010, 69, 85–89. [Google Scholar]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res 2011, 45, 69–86. [Google Scholar]
- Mitchell, J. Streptococcus mitis: Walking the line between commensalism and pathogenesis. Mol. Oral Microbiol 2011, 26, 89–98. [Google Scholar]
- Di Giulio, M.; D’Ercole, S.; Zara, S.; Cataldi, A.; Cellini, L. Streptococcus mitis/human gingival fibroblasts co-culture: The best natural association in answer to the 2-hydroxyethyl methacrylate release. APMIS 2012, 120, 139–146. [Google Scholar]
- Zara, S.; Di Giulio, M.; D’Ercole, S.; Cellini, L.; Cataldi, A. Anti-adhesive and pro-apoptotic effects of 2-hydroxyethyl methacrylate on human gingival fibroblasts co-cultured with Streptococcus mitis strains. Int. Endod. J 2011, 44, 1145–1154. [Google Scholar]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm formation on dental restorative and implant materials. J. Dent. Res 2010, 89, 657–665. [Google Scholar]
- Cheng, L.; Exterkate, R.A.; Zhou, X.; Li, J.; ten Cate, J.M. Effect of Galla chinensis on growth and metabolism of microcosm biofilms. Caries Res 2011, 45, 87–92. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Dieases (ESCMID). Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2000, 6, 503–508.
- D’Ercole, S.; Di Giulio, M.; Grande, R.; Di Campli, E.; Di Bartolomeo, S.; Piccolomini, R.; Cellini, L. Effect of 2-hydroxyethyl methacrylate on Streptococcus spp. biofilms. Lett. Appl. Microbiol. 2011, 52, 193–200. [Google Scholar]
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent. Mater 2012, 28, 219–228. [Google Scholar]


| Streptococcal strains | MIC (% v/v) | MBC (% v/v) | BIC (% v/v) | BEC (% v/v) |
|---|---|---|---|---|
| S. mitis ATCC 6249 | 0.05 | 0.1 | 0.1 | 0.2 |
| S. mutans ATCC 25175 | 0.1 | 0.1 | 0.2 | 0.2 |
| S. oralis ATCC 9811 | 0.1 | 0.1 | 0.2 | 0.2 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Di Giulio, M.; Di Bartolomeo, S.; Di Campli, E.; Sancilio, S.; Marsich, E.; Travan, A.; Cataldi, A.; Cellini, L. The Effect of a Silver Nanoparticle Polysaccharide System on Streptococcal and Saliva-Derived Biofilms. Int. J. Mol. Sci. 2013, 14, 13615-13625. https://doi.org/10.3390/ijms140713615
Di Giulio M, Di Bartolomeo S, Di Campli E, Sancilio S, Marsich E, Travan A, Cataldi A, Cellini L. The Effect of a Silver Nanoparticle Polysaccharide System on Streptococcal and Saliva-Derived Biofilms. International Journal of Molecular Sciences. 2013; 14(7):13615-13625. https://doi.org/10.3390/ijms140713615
Chicago/Turabian StyleDi Giulio, Mara, Soraya Di Bartolomeo, Emanuela Di Campli, Silvia Sancilio, Eleonora Marsich, Andrea Travan, Amelia Cataldi, and Luigina Cellini. 2013. "The Effect of a Silver Nanoparticle Polysaccharide System on Streptococcal and Saliva-Derived Biofilms" International Journal of Molecular Sciences 14, no. 7: 13615-13625. https://doi.org/10.3390/ijms140713615
APA StyleDi Giulio, M., Di Bartolomeo, S., Di Campli, E., Sancilio, S., Marsich, E., Travan, A., Cataldi, A., & Cellini, L. (2013). The Effect of a Silver Nanoparticle Polysaccharide System on Streptococcal and Saliva-Derived Biofilms. International Journal of Molecular Sciences, 14(7), 13615-13625. https://doi.org/10.3390/ijms140713615