Influence of Unmodified and β-Glycerophosphate Cross-Linked Chitosan on Anti-Candida Activity of Clotrimazole in Semi-Solid Delivery Systems
Abstract
:1. Introduction
2. Results and Discussion
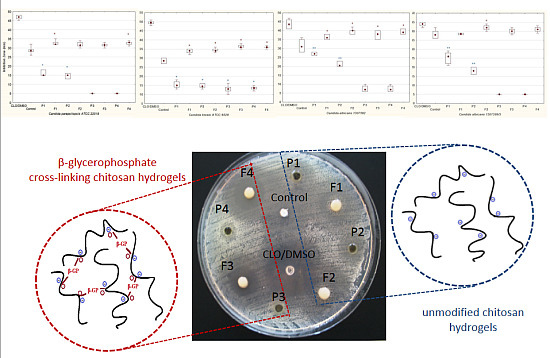
2.1. Anti-Candida Activity of Chitosan Hydrogels
| Formulation | Viscosity (mPa·s) |
|---|---|
| P1 | 17710.5 ± 85.7 |
| P2 | 29302.5 ± 154.2 |
| P3 | 19964.4 ± 114.6 |
| P4 | 19297.6 ± 98.4 |
| F1 | 19987.3 ± 108.2 |
| F2 | 32048.1 ± 134.2 |
| F3 | 20009.4 ± 109.4 |
| F4 | 21828.1 ± 98.7 |


2.2. In Vitro Release of Clotrimazole

3. Experimental Section
3.1. Materials
3.2. Preparation of Hydrogels
3.3. Test Organisms
| Component (g) | Formulation | |||||||
|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | F1 | F2 | F3 | F4 | |
| Clotrimazole | - | - | - | - | 2.0 | 2.0 | 2.0 | 2.0 |
| Chitosan | 3.0 | 4.0 | 3.0 | 4.0 | 3.0 | 4.0 | 3.0 | 4.0 |
| Glycerolum 86% | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Cremophor EL | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| β-GP 45% (w/w) | - | - | 4.2 | 5.6 | - | - | 4.2 | 5.6 |
| d-Glucono-1,5-lactone and sodium benzoate | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1.8% Acetic acid ad | 100.0 | - | 100.0 | - | 100.0 | - | 100.0 | - |
| 2.4% Acetic acid ad | - | 100.0 | - | 100.0 | - | 100.0 | - | 100.0 |
3.4. Plate Diffusion Method
3.5. Hydrogels’ Viscosity Measurements
3.6. HPLC Analysis
3.7. In Vitro Release of Clotrimazole

3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shaji, J.; Jain, V.; Lodha, S. Chitosan: A novel pharmaceutical excipient. Int. J. Pharm. Appl. Sci. 2010, 1, 11–28. [Google Scholar]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Progr. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Ghasemi Tahrir, F.; Ganji, F.; Mani, A.R.; Khodaverdi, E. In vitro and in vivo evaluation of thermosensitive chitosan hydrogel for sustained release of insulin. Drug Deliv. 2014, 9, 1–9. [Google Scholar]
- Zhang, D.; Sun, P.; Li, P.; Xue, A.; Zhang, X.; Zhang, H.; Jin, X. A magnetic chitosan hydrogel for sustained and prolonged delivery of Bacillus Calmette-Guérin in the treatment of bladder cancer. Biomaterials 2013, 34, 10258–10266. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Casettari, L.; Cespi, M.; Palmieri, G.F.; Bonacucina, G. Characterization of the interaction between chitosan and inorganic sodium phosphates by means of rheological and optical microscopy studies. Carbohydr. Polym. 2013, 91, 597–602. [Google Scholar] [CrossRef]
- Ding, K.; Yang, Z.; Zhang, Y.L.; Xu, J.Z. Injectable thermosensitive chitosan/β-glycerophosphate/collagen hydrogel maintains the plasticity of skeletal muscle satellite cells and supports their in vivo viability. Cell. Biol. Int. 2013, 37, 977–987. [Google Scholar] [CrossRef]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Curdy, C.; Vandamme, T. Thermosensitive chitosan/glycerophosphate-based hydrogel and its derivatives in pharmaceutical and biomedical applications. Expert Opin. Drug Deliv. 2014, 11, 249–267. [Google Scholar] [CrossRef]
- Niranjan, R.; Koushik, C.; Saravanan, S.; Moorthi, A.; Vairamani, M.; Selvamurugan, N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2013, 54, 24–29. [Google Scholar] [CrossRef]
- Xie, J.L.; Polvi, E.J.; Shekhar-Guturja, T.; Cowen, L.E. Elucidating drug resistance in human fungal pathogens. Future Microbiol. 2014, 9, 523–542. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic antimicrobial polymers and their assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef]
- Chien, H.F.; Chen, C.P.; Chen, Y.C.; Chang, P.H.; Tsai, T.; Chen, C.T. The use of chitosan to enhance photodynamic inactivation against Candida albicans and its drug-resistant clinical isolates. Int. J. Mol. Sci. 2013, 14, 7445–7456. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Preparation and in vitro evaluation of chitosan microgranules with clotrimazole. Acta Pol. Pharm. Drug Res. 2012, 69, 509–513. [Google Scholar]
- Szymańska, E.; Winnicka, K.; Amelian, A.; Cwalina, U. Vaginal chitosan tablets with clotrimazole-design and evaluation of mucoadhesive properties using porcine vaginal mucosa, mucin and gelatine. Chem. Pharm. Bull. 2014, 62, 160–167. [Google Scholar] [CrossRef]
- Henry, K.W.; Nickels, J.T.; Edlind, T.D. Upregulation of ERG genes in Candida species by azoles and other sterol biosynthesis inhibitors. Antimicrob. Agents Chemother. 2000, 44, 2693–2700. [Google Scholar] [CrossRef]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Simonetti, G.; Baffa, S.; Simonetti, N. Contact imidazole activity against resistant bacteria and fungi. Int. J. Antimicrob. Agents 2001, 17, 389–393. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, A.; Passarinha, L.A.; Gaspar, C.; Palmeira-de-Oliveira, R.; Sarmento, B.; Martinez-de-Oliveira, J.; Pina-Vaz, C.; Rodrigues, A.G.; Queiroz, J.A. The relationship between Candida species charge density and chitosan activity evaluated by ion-exchange chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 3749–3751. [Google Scholar] [CrossRef]
- Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast: Approved Standard, 3rd ed.; CLSI document M27-A3, Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.
- Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast, Third Informational Supplement; CLSI document M27-S3, Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.
- Yancheva, E.; Paneva, D.; Maximova, V.; Mespouille, L.; Dubois, P.; Manolova, N.; Rashkov, I. Polyelectrolyte complexes between (cross-linked) N-carboxyethylchitosan and (quaternized) poly[2-(dimethylamino)ethyl methacrylate]: Preparation, characterization, and antibacterial properties. Biomacromolecules 2007, 8, 976–984. [Google Scholar] [CrossRef]
- Ji, Q.X.; Zhao, Q.S.; Deng, J.; Lv, R. A novel injectable chlorhexidine thermosensitive hydrogel for periodontal application: Preparation, antibacterial activity and toxicity evaluation. J. Mater. Sci. Mater. Med. 2010, 21, 2435–2442. [Google Scholar]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Schoch, C.; Vandamme, T. Rheological study of chitosan/polyol-phosphate systems: Influence of the polyol part on the thermo-induced gelation mechanism. Langmuir 2013, 29, 10229–10237. [Google Scholar] [CrossRef]
- Chien, Y.W.; Chi, H.L. Drug delivery: Vaginal route. In Encyclopedia of Pharmaceutical Technology, 3rd ed.; Swarbrick, J., Ed.; Informa Healthcare: New York, NY, USA, 2007; Volume 2, pp. 1339–1361. [Google Scholar]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination degree of deacetylation of chitosan: Comparison of methods. Prog. Chem. Appl. Chitin Its Deriv. 2012, 17, 5–20. [Google Scholar]
- Rinaudo, M.; Pavlov, G.; Desbrières, J. Influence of acetic acid concentration on the solubilization of chitosan. Polymer 1999, 40, 7029–7032. [Google Scholar] [CrossRef]
- De Hoog, G.S.; Guarro, J. Atlas of Clinical Fungi, 1st ed.; Centraalbureau voor Schimmelcultures: Baarn, Delft, The Netherlands, 1995. [Google Scholar]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. Hydrogel of ketoconazole and PAMAM dendrimers: Formulation and antifungal activity. Molecules 2012, 17, 4612–4624. [Google Scholar] [CrossRef]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. The effect of PAMAM dendrimers on the antibacterial activity of antibiotics with different water solubility. Molecules 2013, 18, 8607–8017. [Google Scholar]
- Sosnowska, K.; Winnicka, K. PAMAM dendrimers affect the in vitro release of clotrimazole from hydrogels irrespective of its molecular state. Afr. J. Pharm. Pharmacol. 2013, 7, 567–573. [Google Scholar] [CrossRef]
- In Vitro Release Testing and In Vivo Bioequivalence Documentation. In Guidance for Industry. SUPAC-SS Non-Sterile Semisolid Dosage Forms. Scale-up and Postapproval Changes: Chemistry, Manufacturing and Controls; FDA-SUPAC-SS: Rockville, MD, USA, 1997; pp. 19–24.
- Siewert, M.; Dressman, J.; Brown, C.K.; Shah, V.P. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech. 2003, 4, 43–52. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska, E.; Winnicka, K.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E.A. Influence of Unmodified and β-Glycerophosphate Cross-Linked Chitosan on Anti-Candida Activity of Clotrimazole in Semi-Solid Delivery Systems. Int. J. Mol. Sci. 2014, 15, 17765-17777. https://doi.org/10.3390/ijms151017765
Szymańska E, Winnicka K, Wieczorek P, Sacha PT, Tryniszewska EA. Influence of Unmodified and β-Glycerophosphate Cross-Linked Chitosan on Anti-Candida Activity of Clotrimazole in Semi-Solid Delivery Systems. International Journal of Molecular Sciences. 2014; 15(10):17765-17777. https://doi.org/10.3390/ijms151017765
Chicago/Turabian StyleSzymańska, Emilia, Katarzyna Winnicka, Piotr Wieczorek, Paweł Tomasz Sacha, and Elżbieta Anna Tryniszewska. 2014. "Influence of Unmodified and β-Glycerophosphate Cross-Linked Chitosan on Anti-Candida Activity of Clotrimazole in Semi-Solid Delivery Systems" International Journal of Molecular Sciences 15, no. 10: 17765-17777. https://doi.org/10.3390/ijms151017765
APA StyleSzymańska, E., Winnicka, K., Wieczorek, P., Sacha, P. T., & Tryniszewska, E. A. (2014). Influence of Unmodified and β-Glycerophosphate Cross-Linked Chitosan on Anti-Candida Activity of Clotrimazole in Semi-Solid Delivery Systems. International Journal of Molecular Sciences, 15(10), 17765-17777. https://doi.org/10.3390/ijms151017765