The Signaling Role of CD40 Ligand in Platelet Biology and in Platelet Component Transfusion
Abstract
:1. Introduction
2. What Is CD40L?

3. What Is the Function of CD40L?
3.1. CD40/CD40L in Physiology
3.2. CD40L and Its Receptors in Inflammatory Pathologies

4. Platelet CD40L

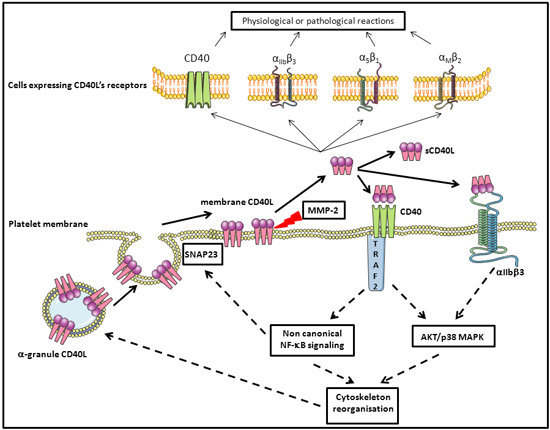
5. Platelets, CD40L, and Molecular Signaling
5.1. Platelet Activation in Platelet Components and Molecular Signaling

5.2. Platelet Membrane CD40L Regulation and Shedding
5.3. Platelet and CD40L Signaling
6. CD40L and Platelet Component Transfusion
7. Concluding Remarks: Towards Molecular Medicine Based upon CD40L and CD40 Polymorphisms
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grewal, I.S.; Flavell, R.A. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 1998, 16, 111–135. [Google Scholar] [PubMed]
- Banchereau, J.; Bazan, F.; Blanchard, D.; Brière, F.; Galizzi, J.P.; van Kooten, C.; Liu, Y.J.; Rousset, F.; Saeland, S. The CD40 antigen and its ligand. Annu. Rev. Immunol. 1994, 12, 881–922. [Google Scholar] [CrossRef] [PubMed]
- Van Kooten, C.; Banchereau, J. CD40-CD40 ligand. J. Leukoc. Biol. 2000, 67, 2–17. [Google Scholar] [PubMed]
- Elgueta, R.; Benson, M.J.; de Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Malarstig, A.; Lindahl, B.; Wallentin, L.; Siegbahn, A. Soluble CD40L levels are regulated by the −3459 A>G polymorphism and predict myocardial infarction and the efficacy of antithrombotic treatment in non-ST elevation acute coronary syndrome. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Phipps, R.P.; Kaufmann, J.; Blumberg, N. Platelet derived CD154 (CD40 ligand) and febrile responses to transfusion. Lancet 2001, 357, 2023–2024. [Google Scholar] [PubMed]
- Blumberg, N.; Gettings, K.F.; Turner, C.; Heal, J.M.; Phipps, R.P. An association of soluble CD40 ligand (CD154) with adverse reactions to platelet transfusions. Transfusion 2006, 46, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, N.; Spinelli, S.L.; Francis, C.W.; Taubman, M.B.; Phipps, R.P. The platelet as an immune cell—CD40 ligand and transfusion immunomodulation. Immunol. Res. 2009, 45, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Cognasse, F.; Boussoulade, F.; Chavarin, P.; Acquart, S.; Fabrigli, P.; Lamy, B.; Garraud, O. Release of potential immunomodulatory factors during platelet storage. Transfusion 2006, 46, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.Y.; Kelher, M.R.; Heal, J.M.; Blumberg, N.; Boshkov, L.K.; Phipps, R.; Gettings, K.F.; McLaughlin, N.J.; Silliman, C.C. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood 2006, 108, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh-Cognasse, H.; Damien, P.; Nguyen, K.A.; Arthaud, C.-A.; Eyraud, M.-A.; Chavarin, P.; Absi, L.; Osselaer, J.-C.; Pozzetto, B.; Cognasse, F.; et al. Immune-reactive soluble OX40 ligand, soluble CD40 ligand, and interleukin-27 are simultaneously oversecreted in platelet components associated with acute transfusion reactions. Transfusion 2014, 54, 613–625. [Google Scholar]
- Nguyen, K.A.; Hamzeh-Cognasse, H.; Sebban, M.; Fromont, E.; Chavarin, P.; Absi, L.; Pozzetto, B.; Cognasse, F.; Garraud, O. A computerized prediction model of hazardous inflammatory platelet transfusion outcomes. PLoS One 2014, 9, e97082. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Bakogiannis, C.; Tousoulis, D.; Antonopoulos, A.S.; Stefanadis, C. The CD40/CD40 ligand system: linking inflammation with atherothrombosis. J. Am. Coll. Cardiol. 2009, 54, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Alaaeddine, N.; Hassan, G.S.; Yacoub, D.; Mourad, W. CD154: An immunoinflammatory mediator in systemic lupus erythematosus and rheumatoid arthritis. Clin. Dev. Immunol. 2012, 2012. [Google Scholar] [CrossRef]
- Dejica, D.I.; Manea, E.M. Costimulatory molecule CD154 in systemic lupus erythematosus and rheumatoid arthritis. Therapeutic perspectives. Roum. Arch. Microbiol. Immunol. 2006, 65, 66–74. [Google Scholar]
- Zhang, B.; Wu, T.; Chen, M.; Zhou, Y.; Yi, D.; Guo, R. The CD40/CD40L system: A new therapeutic target for disease. Immunol. Lett. 2013, 153, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.X.; Viles-Gonzalez, J.F.; Badimon, J.J.; Cavusoglu, E.; Marmur, J.D. Membrane-associated CD40L and sCD40L in atherothrombotic disease. Thromb. Haemost. 2003, 90, 377–384. [Google Scholar] [PubMed]
- Ludewig, B.; Henn, V.; Schröder, J.M.; Graf, D.; Kroczek, R.A. Induction, regulation, and function of soluble TRAP (CD40 ligand) during interaction of primary CD4+ CD45RA+ T cells with dendritic cells. Eur. J. Immunol. 1996, 26, 3137–3143. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.J.; Riley, J.L.; Harlan, D.M.; Abe, R.; Tadaki, D.K.; Hoffmann, S.C.; White, L.; Francomano, T.; Perfetto, S.J.; Kirk, A.D.; et al. CD40 ligand (CD154) triggers a short-term CD4+ T cell activation response that results in secretion of immunomodulatory cytokines and apoptosis. J. Exp. Med. 2000, 191, 651–660. [Google Scholar]
- Graf, D.; Müller, S.; Korthäuer, U.; van Kooten, C.; Weise, C.; Kroczek, R.A. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur. J. Immunol. 1995, 25, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Ensembl Genome Browser 77: Homo Sapiens-Summary-Gene: CD40LG (ENSG00000102245). Available online: http://www.ensembl.org/Homo_sapiens/Gene/Summary?db=core;g=ENSG00000102245;r=X:136648193-136660390 (accessed on 8 October 2014).
- Lievens, D.; Eijgelaar, W.J.; Biessen, E.A.L.; Daemen, M.J.A.P.; Lutgens, E. The multi-functionality of CD40L and its receptor CD40 in atherosclerosis. Thromb. Haemost. 2009, 102, 206–214. [Google Scholar] [PubMed]
- Ma, D.Y.; Clark, E.A. The role of CD40 and CD154/CD40L in dendritic cells. Semin. Immunol. 2009, 21, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Henn, V.; Slupsky, J.R.; Gräfe, M.; Anagnostopoulos, I.; Förster, R.; Müller-Berghaus, G.; Kroczek, R.A. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Inwald, D.P. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ. Res. 2003, 92, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Girvin, A.M.; dal Canto, M.C.; Miller, S.D. CD40/CD40L interaction is essential for the induction of EAE in the absence of CD28-mediated co-stimulation. J. Autoimmun. 2002, 18, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.L.; Wagner, D.H.; Haskins, K. CD40 on NOD CD4 T cells contributes to their activation and pathogenicity. J. Autoimmun. 2008, 31, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Vaitaitis, G.M.; Wagner, D.H. High distribution of CD40 and TRAF2 in Th40 T cell rafts leads to preferential survival of this auto-aggressive population in autoimmunity. PLoS One 2008, 3, e2076. [Google Scholar] [CrossRef] [PubMed]
- Munroe, M.E. Functional roles for T cell CD40 in infection and autoimmune disease: The role of CD40 in lymphocyte homeostasis. Semin. Immunol. 2009, 21, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Tone, M.; Tone, Y.; Fairchild, P.J.; Wykes, M.; Waldmann, H. Regulation of CD40 function by its isoforms generated through alternative splicing. Proc. Natl. Acad. Sci. USA 2001, 98, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Obregon, D.; Lou, D.; Ehrhart, J.; Fernandez, F.; Silver, A.; Tan, J. Modulation of neuronal differentiation by CD40 isoforms. Biochem. Biophys. Res. Commun. 2008, 369, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Chatzigeorgiou, A.E.; Lembessis, P.E.; Mylona-Karagianni, C.F.; Tsouvalas, E.A.; Diamanti-Kandarakis, E.; Kamper, E.F. CD40 expression and its association with low-grade inflammation in a Greek population of type 1 diabetic juveniles: Evidence for differences in CD40 mRNA isoforms expressed by peripheral blood mononuclear cells. Exp. Clin. Endocrinol. Diabetes 2010, 118, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Contin, C.; Pitard, V.; Itai, T.; Nagata, S.; Moreau, J.-F.; Déchanet-Merville, J. Membrane-anchored CD40 is processed by the tumor necrosis factor-alpha-converting enzyme. Implications for CD40 signaling. J. Biol. Chem. 2003, 278, 32801–32809. [Google Scholar]
- Eshel, D.; Toporik, A.; Efrati, T.; Nakav, S.; Chen, A.; Douvdevani, A. Characterization of natural human antagonistic soluble CD40 isoforms produced through alternative splicing. Mol. Immunol. 2008, 46, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Esposito, P.; Rampino, T.; dal Canton, A. Soluble CD40 as a modulator of CD40 pathway. Immunol. Lett. 2012, 147, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Moreno, C.; Girouard, J.; Lapointe, R.; Darveau, A.; Mourad, W. CD40/CD40 homodimers are required for CD40-induced phosphatidylinositol 3-kinase-dependent expression of B7.2 by human B lymphocytes. J. Biol. Chem. 2004, 279, 7799–7806. [Google Scholar]
- Pullen, S.S.; Labadia, M.E.; Ingraham, R.H.; McWhirter, S.M.; Everdeen, D.S.; Alber, T.; Crute, J.J.; Kehry, M.R. High-affinity interactions of tumor necrosis factor receptor-associated factors (TRAFs) and CD40 require TRAF trimerization and CD40 multimerization. Biochemistry 1999, 38, 10168–10177. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, L.; Liu, Y. Enhancement of binding activity of soluble human CD40 to CD40 ligand through incorporation of an isoleucine zipper motif. Acta Pharmacol. Sin. 2006, 27, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Fullard, J.F. The role of the platelet glycoprotein IIb/IIIa in thrombosis and haemostasis. Curr. Pharm. Des. 2004, 10, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, R.M.; Rose, J.W.; Hsu, M.A.; Phillips, D.R.; Fried, V.A.; Campbell, A.M.; Nannizzi, L.; Charo, I.F. Barbourin. A GPIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J. Biol. Chem. 1991, 266, 9359–9362. [Google Scholar]
- Zirlik, A.; Maier, C.; Gerdes, N.; MacFarlane, L.; Soosairajah, J.; Bavendiek, U.; Ahrens, I.; Ernst, S.; Bassler, N.; Missiou, A.; et al. CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation 2007, 115, 1571–1580. [Google Scholar]
- Léveillé, C.; Bouillon, M.; Guo, W.; Bolduc, J.; Sharif-Askari, E.; el-Fakhry, Y.; Reyes-Moreno, C.; Lapointe, R.; Merhi, Y.; Wilkins, J.A.; et al. CD40 ligand binds to alpha5beta1 integrin and triggers cell signaling. J. Biol. Chem. 2007, 282, 5143–5151. [Google Scholar]
- El Fakhry, Y.; Alturaihi, H.; Yacoub, D.; Liu, L.; Guo, W.; Leveillé, C.; Jung, D.; Khzam, L.B.; Merhi, Y.; Wilkins, J.A.; et al. Functional interaction of CD154 protein with α5β1 integrin is totally independent from its binding to αIIbβ3 integrin and CD40 molecules. J. Biol. Chem. 2012, 287, 18055–18066. [Google Scholar]
- Hassan, G.S.; Merhi, Y.; Mourad, W.M. CD154 and its receptors in inflammatory vascular pathologies. Trends Immunol. 2009, 30, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, J.A.; Shu, G.; Gallagher, M.; Clark, E.A. Augmentation of normal and malignant B cell proliferation by monoclonal antibody to the B cell-specific antigen BP50 (CDW40). J. Immunol. 1987, 138, 788–794. [Google Scholar] [PubMed]
- Durandy, A.; Kracker, S.; Fischer, A. Primary antibody deficiencies. Nat. Rev. Immunol. 2013, 13, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Nutt, S.L.; Tarlinton, D.M. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat. Immunol. 2011, 12, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Baumjohann, D.; Preite, S.; Reboldi, A.; Ronchi, F.; Ansel, K.M.; Lanzavecchia, A.; Sallusto, F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 2013, 38, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, R.; Gigley, J.P.; Khan, I.A. Cutting edge: CD40-CD40 ligand pathway plays a critical CD8-intrinsic and -extrinsic role during rescue of exhausted CD8 T cells. J. Immunol. 2011, 187, 4421–4425. [Google Scholar] [CrossRef] [PubMed]
- Korniluk, A.; Kemona, H.; Dymicka-Piekarska, V. Multifunctional CD40L: Pro- and anti-neoplastic activity. Tumour Biol. 2014, 35, 9447–9457. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Miszti-Blasius, K.; Kerenyi, A.; Clemetson, K.J.; Kappelmayer, J. Potential therapeutic targeting of platelet-mediated cellular interactions in atherosclerosis and inflammation. Curr. Med. Chem. 2012, 19, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.S.S.; Andre, P.; Yan, Y.; Phillips, D.R. The platelet CD40L/GP IIb-IIIa axis in atherothrombotic disease. Curr. Opin. Hematol. 2003, 10, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Pamukcu, B.; Lip, G.Y.H.; Snezhitskiy, V.; Shantsila, E. The CD40-CD40L system in cardiovascular disease. Ann. Med. 2011, 43, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Lievens, D.; Zernecke, A.; Seijkens, T.; Soehnlein, O.; Beckers, L.; Munnix, I.C.A.; Wijnands, E.; Goossens, P.; van Kruchten, R.; Thevissen, L.; et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood 2010, 116, 4317–4327. [Google Scholar]
- Nurden, A.T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 2011, 105 (Suppl. 1), S13–S33. [Google Scholar] [CrossRef]
- Sanguigni, V.; Ferro, D.; Pignatelli, P.; del Ben, M.; Nadia, T.; Saliola, M.; Sorge, R.; Violi, F. CD40 ligand enhances monocyte tissue factor expression and thrombin generation via oxidative stress in patients with hypercholesterolemia. J. Am. Coll. Cardiol. 2005, 45, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; van der Poll, T.; Büller, H.R. Bidirectional relation between inflammation and coagulation. Circulation 2004, 109, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Yaron, R.; Yellin, M.J. CD40L-CD40 interactions regulate endothelial cell surface tissue factor and thrombomodulin expression. J. Leukoc. Biol. 1998, 63, 373–379. [Google Scholar] [PubMed]
- Chen, Y.; Chen, J.; Xiong, Y.; Da, Q.; Xu, Y.; Jiang, X.; Tang, H. Internalization of CD40 regulates its signal transduction in vascular endothelial cells. Biochem. Biophys. Res. Commun. 2006, 345, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chai, H.; Wang, X.; Jiang, J.; Jamaluddin, M.S.; Liao, D.; Zhang, Y.; Wang, H.; Bharadwaj, U.; Zhang, S.; et al. Soluble CD40 ligand induces endothelial dysfunction in human and porcine coronary artery endothelial cells. Blood 2008, 112, 3205–3216. [Google Scholar]
- Gururajan, P.; Gurumurthy, P.; Nayar, P.; Babu, S.; Sarasabharati, A.; Victor, D.; Cherian, K.M. Increased serum concentrations of Soluble CD40 Ligand as a prognostic marker in patients with Acute Coronary Syndrome. Indian J. Clin. Biochem. 2009, 24, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, X.; Mannon, R.B.; Kirk, A.D. Platelet-derived or soluble CD154 induces vascularized allograft rejection independent of cell-bound CD154. J. Clin. Investig. 2006, 116, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Toubi, E.; Shoenfeld, Y. The role of CD40-CD154 interactions in autoimmunity and the benefit of disrupting this pathway. Autoimmunity 2004, 37, 457–464. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Nannizzi-Alaimo, L.; Prasad, S.K.; Phillips, D.R. Platelet-derived CD40L: The switch-hitting player of cardiovascular disease. Circulation 2002, 106, 896–899. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Prasad, K.S.S.; Denis, C.V.; He, M.; Papalia, J.M.; Hynes, R.O.; Phillips, D.R.; Wagner, D.D. CD40L stabilizes arterial thrombi by a beta3 integrin-dependent mechanism. Nat. Med. 2002, 8, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.S.; Andre, P.; He, M.; Bao, M.; Manganello, J.; Phillips, D.R. Soluble CD40 ligand induces β3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 12367–12371. [Google Scholar] [CrossRef] [PubMed]
- May, A.E.; Kälsch, T.; Massberg, S.; Herouy, Y.; Schmidt, R.; Gawaz, M. Engagement of glycoprotein IIb/IIIa (α(IIb)β3) on platelets upregulates CD40L and triggers CD40L-dependent matrix degradation by endothelial cells. Circulation 2002, 106, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Varghese, S.; Vitseva, O.; Tanriverdi, K.; Freedman, J.E. CD40 ligand influences platelet release of reactive oxygen intermediates. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sanders, J.M.; Bevard, M.H.; Sun, Z.; Chumley, J.W.; Galkina, E.V.; Ley, K.; Sarembock, I.J. CD40 ligand promotes Mac-1 expression, leukocyte recruitment, and neointima formation after vascular injury. Am. J. Pathol. 2008, 172, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Tung, J.-P.; Fraser, J.F.; Nataatmadja, M.; Colebourne, K.I.; Barnett, A.G.; Glenister, K.M.; Zhou, A.Y.; Wood, P.; Silliman, C.C.; Fung, Y.L. Age of blood and recipient factors determine the severity of transfusion-related acute lung injury (TRALI). Crit. Care 2012, 16. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Zhang, S.; Chew, M.; Ersson, A.; Jeppsson, B.; Thorlacius, H. Platelet-derived CD40L (CD154) mediates neutrophil upregulation of Mac-1 and recruitment in septic lung injury. Ann. Surg. 2009, 250, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Vlaar, A.P.J.; Juffermans, N.P. Transfusion-related acute lung injury: a clinical review. Lancet 2013, 382, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Chang, J.; Jang, J.-E.; Peired, A.J.; Chiang, E.Y.; Frenette, P.S. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat. Med. 2009, 15, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.S.; Merhi, Y.; Mourad, W. CD40 ligand: A neo-inflammatory molecule in vascular diseases. Immunobiology 2012, 217, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Hermann, A. Platelet CD40 ligand (CD40L)—subcellular localization, regulation of expression, and inhibition by clopidogrel. Platelets 2001, 12, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Sans, M.; Fiocchi, C. The CD40/CD40L costimulatory pathway in inflammatory bowel disease. Gut 2004, 53, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Charafeddine, A.H.; Kim, E.J.; Maynard, D.M.; Yi, H.; Weaver, T.A.; Gunay-Aygun, M.; Russell, M.; Gahl, W.A.; Kirk, A.D. Platelet-derived CD154: Ultrastructural localization and clinical correlation in organ transplantation. Am. J. Transplant. 2012, 12, 3143–3151. [Google Scholar] [CrossRef] [PubMed]
- Doescher, A.; Petershofen, E.K.; Hertenstein, B.; Kraemer, D.; Casper, J.; Schmidt, J.-P.; Müller, T.H. Platelet recovery and survival measured in patients by quantitative polymerase chain reaction of mitochondrial DNA. Transfusion 2014. [Google Scholar] [CrossRef]
- Denis, M.M.; Tolley, N.D.; Bunting, M.; Schwertz, H.; Jiang, H.; Lindemann, S.; Yost, C.C.; Rubner, F.J.; Albertine, K.H.; Swoboda, K.J.; et al. Escaping the nuclear confines: Signal-dependent pre-mRNA splicing in anucleate platelets. Cell 2005, 122, 379–391. [Google Scholar]
- Nurden, A.T.; Nurden, P.; Sanchez, M.; Andia, I.; Anitua, E. Platelets and wound healing. Front. Biosci. J. Virtual Libr. 2008, 13, 3532–3548. [Google Scholar]
- Plé, H.; Maltais, M.; Corduan, A.; Rousseau, G.; Madore, F.; Provost, P. Alteration of the platelet transcriptome in chronic kidney disease. Thromb. Haemost. 2012, 108, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Garraud, O.; Berthet, J.; Hamzeh-Cognasse, H.; Cognasse, F. Pathogen sensing, subsequent signalling, and signalosome in human platelets. Thromb. Res. 2011, 127, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Gnatenko, D.V.; Dunn, J.J.; McCorkle, S.R.; Weissmann, D.; Perrotta, P.L.; Bahou, W.F. Transcript profiling of human platelets using microarray and serial analysis of gene expression. Blood 2003, 101, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.W.; Oler, A.J.; Tolley, N.D.; Hunter, B.N.; Low, E.N.; Nix, D.A.; Yost, C.C.; Zimmerman, G.A.; Weyrich, A.S. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood 2011, 118, e101–e111. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.M.; Edelstein, L.C.; Nagalla, S.; Woodley, A.B.; Chen, E.S.; Kong, X.; Ma, L.; Fortina, P.; Kunapuli, S.; Holinstat, M.; et al. Human platelet microRNA-mRNA networks associated with age and gender revealed by integrated plateletomics. Blood 2014, 123, e37–e45. [Google Scholar]
- Nagalla, S.; Shaw, C.; Kong, X.; Kondkar, A.A.; Edelstein, L.C.; Ma, L.; Chen, J.; McKnight, G.S.; López, J.A.; Yang, L.; et al. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood 2011, 117, 5189–5197. [Google Scholar]
- Flaumenhaft, R. Molecular basis of platelet granule secretion. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Rendu, F.; Brohard-Bohn, B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets 2001, 12, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet α-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef]
- Jackson, S.P.; Nesbitt, W.S.; Westein, E. Dynamics of platelet thrombus formation. J. Thromb. Haemost. 2009, 7, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Henn, V.; Steinbach, S.; Büchner, K.; Presek, P.; Kroczek, R.A. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood 2001, 98, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Elzey, B.D.; Grant, J.F.; Sinn, H.W.; Nieswandt, B.; Waldschmidt, T.J.; Ratliff, T.L. Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation. J. Leukoc. Biol. 2005, 78, 80–84. [Google Scholar] [PubMed]
- Elzey, B.D.; Tian, J.; Jensen, R.J.; Swanson, A.K.; Lees, J.R.; Lentz, S.R.; Stein, C.S.; Nieswandt, B.; Wang, Y.; Davidson, B.L.; et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity 2003, 19, 9–19. [Google Scholar]
- Cognasse, F.; Hamzeh-Cognasse, H.; Lafarge, S.; Chavarin, P.; Cogné, M.; Richard, Y.; Garraud, O. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp. Hematol. 2007, 35, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Leroyer, A.S.; Rautou, P.-E.; Silvestre, J.-S.; Castier, Y.; Lesèche, G.; Devue, C.; Duriez, M.; Brandes, R.P.; Lutgens, E.; Tedgui, A.; et al. CD40 ligand+ microparticles from human atherosclerotic plaques stimulate endothelial proliferation and angiogenesis a potential mechanism for intraplaque neovascularization. J. Am. Coll. Cardiol. 2008, 52, 1302–1311. [Google Scholar]
- Danese, S.; Katz, J.A.; Saibeni, S.; Papa, A.; Gasbarrini, A.; Vecchi, M.; Fiocchi, C. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut 2003, 52, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Viallard, J.-F.; Solanilla, A.; Gauthier, B.; Contin, C.; Déchanet, J.; Grosset, C.; Moreau, J.-F.; Praloran, V.; Nurden, P.; Pellegrin, J.-L.; et al. Increased soluble and platelet-associated CD40 ligand in essential thrombocythemia and reactive thrombocytosis. Blood 2002, 99, 2612–2614. [Google Scholar]
- Chaturvedi, R.; Gupta, M.; Jain, A.; Das, T.; Prashar, S. Soluble CD40 ligand: A novel biomarker in the pathogenesis of periodontal disease. Clin. Oral Investig. 2014. [Google Scholar] [CrossRef]
- Galicia López, A.; Olguín Ortega, L.; Saavedra, M.A.; Méndez Cruz, R.; Jimenez Flores, R.; García de la Peña, M. Increased concentrations of soluble CD40 ligand platelet in patients with primary antiphospholipidic syndrome. Reumatol. Clin. 2013, 9, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Kim, H.-S.; Youn, J.-C.; Shin, E.-C.; Park, S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J. Transl. Med. 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [PubMed]
- Varga-Szabo, D.; Pleines, I.; Nieswandt, B. Cell Adhesion mechanisms in platelets. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Lozano, M. L.; Navarro-Núñez, L.; Vicente, V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica 2009, 94, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Thon, J.N.; Schubert, P.; Devine, D.V. Platelet storage lesion: A new understanding from a proteomic perspective. Transfus. Med. Rev. 2008, 22, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Dzieciatkowska, M.; D’Alessandro, A.; Burke, T.A.; Kelher, M.R.; Moore, E.E.; Banerjee, A.; Silliman, C.C.; West, B.F.; Hansen, K.C. Proteomics of apheresis platelet supernatants during routine storage: Gender-related differences. J. Proteomics 2014, 112C, 190–209. [Google Scholar]
- Ohto, H.; Nollet, K.E. Overview on platelet preservation: Better controls over storage lesion. Transfus. Apher. Sci. 2011, 44, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Estebanell, E.; Díaz-Ricart, M.; Escolar, G.; Lozano, M.; Mazzara, R.; Ordinas, A. Alterations in cytoskeletal organization and tyrosine phosphorylation in platelet concentrates prepared by the buffy coat method. Transfusion 2000, 40, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Schubert, P.; Thon, J.N.; Walsh, G.M.; Chen, C.H.I.; Moore, E.D.; Devine, D.V.; Kast, J. A signaling pathway contributing to platelet storage lesion development: targeting PI3-kinase-dependent Rap1 activation slows storage-induced platelet deterioration. Transfusion 2009, 49, 1944–1955. [Google Scholar] [PubMed]
- Canault, M.; Duerschmied, D.; Brill, A.; Stefanini, L.; Schatzberg, D.; Cifuni, S.M.; Bergmeier, W.; Wagner, D.D. p38 mitogen-activated protein kinase activation during platelet storage: Consequences for platelet recovery and hemostatic function in vivo. Blood 2010, 115, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Escolar, G.; White, J.G. Changes in glycoprotein expression after platelet activation: Differences between in vitro and in vivo studies. Thromb. Haemost. 2000, 83, 371–386. [Google Scholar] [PubMed]
- Kasirer-Friede, A.; Kahn, M.L.; Shattil, S.J. Platelet integrins and immunoreceptors. Immunol. Rev. 2007, 218, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, Y.; Doi, T.; Matsushima-Nishiwaki, R.; Iida, Y.; Akamatsu, S.; Kondo, A.; Kuroyanagi, G.; Yamamoto, N.; Mizutani, J.; Otsuka, T.; et al. Involvement of Rac in thromboxane A2-induced human platelet activation: Regulation of sCD40 ligand release and PDGF-AB secretion. Mol. Med. Rep. 2014. [Google Scholar] [CrossRef]
- Panzer, S. Differential response to LPS isotypes induced platelet activation mediated by Toll-like receptor (TLR)-4. Clin. Immunol. 2013, 146, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Rivadeneyra, L.; Carestia, A.; Etulain, J.; Pozner, R.G.; Fondevila, C.; Negrotto, S.; Schattner, M. Regulation of platelet responses triggered by Toll-like receptor 2 and 4 ligands is another non-genomic role of nuclear factor-κB. Thromb. Res. 2014, 133, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Berthet, J.; Damien, P.; Hamzeh-Cognasse, H.; Arthaud, C.-A.; Eyraud, M.-A.; Zéni, F.; Pozzetto, B.; McNicol, A.; Garraud, O.; Cognasse, F. Human platelets can discriminate between various bacterial LPS isoforms via TLR4 signaling and differential cytokine secretion. Clin. Immunol. 2012, 145, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Otterdal, K.; Pedersen, T.M.; Solum, N.O. Release of soluble CD40 ligand after platelet activation: Studies on the solubilization phase. Thromb. Res. 2004, 114, 167–177. [Google Scholar] [PubMed]
- Jin, Y.; Nonoyama, S.; Morio, T.; Imai, K.; Ochs, H.D.; Mizutani, S. Characterization of soluble CD40 ligand released from human activated platelets. J. Med. Dent. Sci. 2001, 48, 23–27. [Google Scholar] [PubMed]
- Mason, P.J.; Chakrabarti, S.; Albers, A.A.; Rex, S.; Vitseva, O.; Varghese, S.; Freedman, J.E. Plasma, serum, and platelet expression of CD40 ligand in adults with cardiovascular disease. Am. J. Cardiol. 2005, 96, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Santos-Martínez, M.J.; Medina, C.; Jurasz, P.; Radomski, M.W. Role of metalloproteinases in platelet function. Thromb. Res. 2008, 121, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Matthies, K.M.G.; Newman, J.L.; Hodzic, A.; Wingett, D.G. Differential regulation of soluble and membrane CD40L proteins in T cells. Cell. Immunol. 2006, 241, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, D.; Benslimane, N.; Al-Zoobi, L.; Hassan, G.; Nadiri, A.; Mourad, W. CD154 is released from T-cells by a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) and ADAM17 in a CD40 protein-dependent manner. J. Biol. Chem. 2013, 288, 36083–36093. [Google Scholar] [CrossRef] [PubMed]
- Furman, M.I.; Krueger, L.A.; Linden, M.D.; Barnard, M.R.; Frelinger, A.L.; Michelson, A.D. Release of soluble CD40L from platelets is regulated by glycoprotein IIb/IIIa and actin polymerization. J. Am. Coll. Cardiol. 2004, 43, 2319–2325. [Google Scholar] [CrossRef] [PubMed]
- Reinboldt, S.; Wenzel, F.; Rauch, B.H.; Hohlfeld, T.; Grandoch, M.; Fischer, J.W.; Weber, A.-A. Preliminary evidence for a matrix metalloproteinase-2 (MMP-2)-dependent shedding of soluble CD40 ligand (sCD40L) from activated platelets. Platelets 2009, 20, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, F.; Rox, J.; Reinboldt, S.; Weber, A.A.; Giers, G.; Fischer, J. Release of soluble CD40L by matrix metalloprotease-2 (MMP-2)-dependent shedding of platelets and its subsequent accumulation in stem cell products of autologous donors. J. Stem Cells Regen. Med. 2010, 6, 66–67. [Google Scholar] [PubMed]
- Choi, W.S.; Jeon, O.H.; Kim, D.S. CD40 ligand shedding is regulated by interaction between matrix metalloproteinase-2 and platelet integrin α(IIb)β(3). J. Thromb. Haemost. 2010, 8, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Roller, J.; Zhang, S.; Syk, I.; Menger, M.D.; Jeppsson, B.; Thorlacius, H. Metalloproteinases regulate CD40L shedding from platelets and pulmonary recruitment of neutrophils in abdominal sepsis. Inflamm. Res. 2012, 61, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Zhang, S.; Chew, M.; Syk, I.; Jeppsson, B.; Thorlacius, H. Platelet shedding of CD40L is regulated by matrix metalloproteinase-9 in abdominal sepsis. J. Thromb. Haemost. 2013, 11, 1385–1398. [Google Scholar] [CrossRef] [PubMed]
- Menchén, L.; Marín-Jiménez, I.; Arias-Salgado, E.G.; Fontela, T.; Hernández-Sampelayo, P.; Rodríguez, M.C.G.; Butta, N.V. Matrix metalloproteinase 9 is involved in Crohn’s disease-associated platelet hyperactivation through the release of soluble CD40 ligand. Gut 2009, 58, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Fernández Bello, I.; Álvarez, M.T.; López-Longo, F.J.; Arias-Salgado, E.G.; Martín, M.; Jiménez-Yuste, V.; Rodríguez de la Rúa, A.; Butta, N.V. Platelet soluble CD40L and matrix metalloproteinase 9 activity are proinflammatory mediators in Behçet disease patients. Thromb. Haemost. 2012, 107, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Nannizzi-Alaimo, L.; Alves, V.L.; Phillips, D.R. Inhibitory effects of glycoprotein IIb/IIIa antagonists and aspirin on the release of soluble CD40 ligand during platelet stimulation. Circulation 2003, 107, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, D.; Hachem, A.; Théorêt, J.-F.; Gillis, M.-A.; Mourad, W.; Merhi, Y. Enhanced levels of soluble CD40 ligand exacerbate platelet aggregation and thrombus formation through a CD40-dependent tumor necrosis factor receptor-associated factor-2/Rac1/p38 mitogen-activated protein kinase signaling pathway. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2424–2433. [Google Scholar] [CrossRef] [PubMed]
- Hachem, A.; Yacoub, D.; Zaid, Y.; Mourad, W.; Merhi, Y. Involvement of nuclear factor κB in platelet CD40 signaling. Biochem. Biophys. Res. Commun. 2012, 425, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Karim, Z.A.; Zhang, J.; Banerjee, M.; Chicka, M.C.; Al Hawas, R.; Hamilton, T.R.; Roche, P.A.; Whiteheart, S.W. IκB kinase phosphorylation of SNAP-23 controls platelet secretion. Blood 2013, 121, 4567–4574. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Ye, S.; Whiteheart, S.W. The platelet release reaction: Just when you thought platelet secretion was simple. Curr. Opin. Hematol. 2008, 15, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Broos, K.; Feys, H.B.; de Meyer, S.F.; Vanhoorelbeke, K.; Deckmyn, H. Platelets at work in primary hemostasis. Blood Rev. 2011, 25, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Vamvakas, E.C.; Blajchman, M.A. Prestorage versus poststorage white cell reduction for the prevention of the deleterious immunomodulatory effects of allogeneic blood transfusion. Transfus. Med. Rev. 2000, 14, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, S.; Wu, X.; Chen, Y.; Song, X.; Jin, Z.; Li, H.; Zhou, Y.; Chen, F.; Huo, Y. Multimarker approach for the prediction of cardiovascular events in patients with mild to moderate coronary artery lesions. A 3-year follow-up study. Int. Heart. J. 2012, 53, 85–90. [Google Scholar]
- Zhao, W.; Zhang, F.; Li, Z.; Yu, H.; Li, Z.; Gao, W. Soluble CD40 ligand is associated with angiographic severity of coronary artery disease in patients with acute coronary syndrome. Chin. Med. J. 2014, 127, 2218–2221. [Google Scholar] [PubMed]
- Gerdes, S.; Osadtschy, S.; Buhles, N.; Baurecht, H.; Mrowietz, U. Cardiovascular biomarkers in patients with psoriasis. Exp. Dermatol. 2014, 23, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Zahn, D.; Petrak, F.; Uhl, I.; Juckel, G.; Neubauer, H.; Hägele, A.-K.; Wiltfang, J.; Herpertz, S. New pathways of increased cardiovascular risk in depression: a pilot study on the association of high-sensitivity C-reactive protein with pro-atherosclerotic markers in patients with depression. J. Affect. Disord. 2013, 146, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Riondino, S.; Vazzana, N.; Santoro, N.; Guadagni, F.; Davì, G. Biomarkers of platelet activation in acute coronary syndromes. Thromb. Haemost. 2012, 108, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V.; Scatena, A.; Migliori, M.; Marchetti, V.; Paoletti, S.; Beati, S. Biomarkers of chronic inflammatory state in uremia and cardiovascular disease. Int. J. Inflamm. 2012, 2012. [Google Scholar] [CrossRef]
- Devine, D.V.; Serrano, K. The platelet storage lesion. Clin. Lab. Med. 2010, 30, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, M. The platelet storage lesion. Transfus. Apher. Sci. 2009, 41, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Cognasse, F.; Hamzeh-Cognasse, H.; Lafarge, S.; Acquart, S.; Chavarin, P.; Courbil, R.; Fabrigli, P.; Garraud, O. Donor platelets stored for at least 3 days can elicit activation marker expression by the recipient’s blood mononuclear cells: An in vitro study. Transfusion 2009, 49, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh-Cognasse, H.; Cognasse, F.; Palle, S.; Chavarin, P.; Olivier, T.; Delézay, O.; Pozzetto, B.; Garraud, O. Direct contact of platelets and their released products exert different effects on human dendritic cell maturation. BMC Immunol. 2008, 9. [Google Scholar] [CrossRef]
- National Agency of Security of the Drug and Health Products. Available online: http://ansm.sante.fr/Mediatheque/Publications/Informations-recentes (accessed on 2 December 2014).
- Tuinman, P.R.; Gerards, M.C.; Jongsma, G.; Vlaar, A.P.; Boon, L.; Juffermans, N.P. Lack of evidence of CD40 ligand involvement in transfusion-related acute lung injury. Clin. Exp. Immunol. 2011, 165, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mody, M.; Herst, R.; Sher, G.; Freedman, J. Flow cytometric analysis of platelet function in stored platelet concentrates. Transfus. Sci. 1999, 20, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Aloui, C.; Sut, C.; Prigent, A.; Fagan, J.; Cognasse, F.; Granados-Herbepin, V.; Touraine, R.; Pozzetto, B.; Aouni, M.; Fendri, C.; et al. Genotyping of polymorphisms responsible for the regulation of the expression of CD40 ligand in two blood donor populations (Auvergne-Loire, France; Sousse and Monastir, Tunisia). Transfus. Clin. Biol. 2013, 20, 293–294. [Google Scholar]
- Tanaka, S.; Hayashi, T.; Tani, Y.; Hirayama, F. Removal by adsorbent beads of biological response modifiers released from platelets, accumulated during storage, and potentially associated with platelet transfusion reactions. Transfusion 2010, 50, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Hayashi, T.; Tani, Y.; Hirayama, F. Removal of biological response modifiers associated with platelet transfusion reactions by columns containing adsorption beads. Transfusion 2014, 54, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Couzin, J. Magnificent obsession. Science 2005, 307, 1712–1715. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloui, C.; Prigent, A.; Sut, C.; Tariket, S.; Hamzeh-Cognasse, H.; Pozzetto, B.; Richard, Y.; Cognasse, F.; Laradi, S.; Garraud, O. The Signaling Role of CD40 Ligand in Platelet Biology and in Platelet Component Transfusion. Int. J. Mol. Sci. 2014, 15, 22342-22364. https://doi.org/10.3390/ijms151222342
Aloui C, Prigent A, Sut C, Tariket S, Hamzeh-Cognasse H, Pozzetto B, Richard Y, Cognasse F, Laradi S, Garraud O. The Signaling Role of CD40 Ligand in Platelet Biology and in Platelet Component Transfusion. International Journal of Molecular Sciences. 2014; 15(12):22342-22364. https://doi.org/10.3390/ijms151222342
Chicago/Turabian StyleAloui, Chaker, Antoine Prigent, Caroline Sut, Sofiane Tariket, Hind Hamzeh-Cognasse, Bruno Pozzetto, Yolande Richard, Fabrice Cognasse, Sandrine Laradi, and Olivier Garraud. 2014. "The Signaling Role of CD40 Ligand in Platelet Biology and in Platelet Component Transfusion" International Journal of Molecular Sciences 15, no. 12: 22342-22364. https://doi.org/10.3390/ijms151222342
APA StyleAloui, C., Prigent, A., Sut, C., Tariket, S., Hamzeh-Cognasse, H., Pozzetto, B., Richard, Y., Cognasse, F., Laradi, S., & Garraud, O. (2014). The Signaling Role of CD40 Ligand in Platelet Biology and in Platelet Component Transfusion. International Journal of Molecular Sciences, 15(12), 22342-22364. https://doi.org/10.3390/ijms151222342