Membrane Trafficking in the Yeast Saccharomyces cerevisiae Model
Abstract
:1. The Yeast Saccharomyces cerevisiae
1.1. Generalities and Historical View

1.2. Saccharomyces cerevisiae as a Model Organism

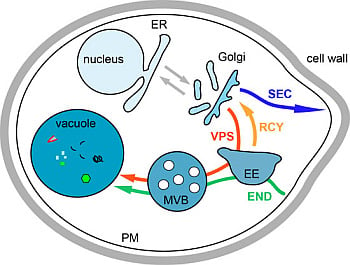
2. Introduction on Membrane Trafficking
3. Biosynthesis Pathway
3.1. Endoplasmic Reticulum to Golgi Transport

3.2. Transport from the Golgi to the Plasma Membrane
3.3. Transport from the Golgi to the Vacuole via the Endosomes, the VPS Pathway
3.3.1. Vacuolar Targeting of Soluble Proteins
3.3.2. Vacuolar Targeting of Membrane Proteins via Multivesicular Body (MVB) Sorting
3.4. Transport from the Golgi to the Vacuole via the AP-3 Adaptor or ALP Pathway
4. Endocytosis

5. The Recycling Pathway
5.1. Recycling of the SNARE Snc1
5.2. Recycling of the Chitin Synthase Chs1 and Chs3
6. New Trafficking Pathways
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Greig, D.; Leu, J.Y. Natural history of budding yeast. Curr. Biol. 2009, 19, R886–R890. [Google Scholar] [CrossRef]
- Walther, A.; Hesselbart, A.; Wendland, J. Genome sequence of saccharomyces carlsbergensis, the worldʼs first pure culture lager yeast. G3 2014, 4, 783–793. [Google Scholar] [CrossRef]
- Amberg, D.C.; Burke, D.J.; Strathern, J.N. Methods in Yeast Genetics, 2005 ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2005. [Google Scholar]
- Goffeau, A.; Barrell, B.G.; Bussey, H.; Davis, R.W.; Dujon, B.; Feldmann, H.; Galibert, F.; Hoheisel, J.D.; Jacq, C.; Johnston, M.; et al. Life with 6000 genes. Science 1996, 274, 563–547. [Google Scholar]
- Sikorski, R.S.; Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in saccharomyces cerevisiae. Genetics 1989, 122, 19–27. [Google Scholar]
- Bard, F.; Malhotra, V. The formation of tgn-to-plasma-membrane transport carriers. Annu. Rev. Cell Dev. Biol. 2006, 22, 439–455. [Google Scholar] [CrossRef]
- Conibear, E. Converging views of endocytosis in yeast and mammals. Curr. Opin. Cell Biol. 2010, 22, 513–518. [Google Scholar] [CrossRef]
- Dancourt, J.; Barlowe, C. Protein sorting receptors in the early secretory pathway. Annu. Rev. Biochem. 2010, 79, 777–802. [Google Scholar] [CrossRef]
- Muniz, M.; Zurzolo, C. Sorting of gpi-anchored proteins from yeast to mammals—Common pathways at different sites? J. Cell Sci. 2014, 127, 2793–2801. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Miller, E.A.; Goldberg, J.; Orci, L.; Schekman, R. Bi-directional protein transport between the er and golgi. Annu. Rev. Cell Dev. Biol. 2004, 20, 87–123. [Google Scholar] [PubMed]
- Barlowe, C.K.; Miller, E.A. Secretory protein biogenesis and traffic in the early secretory pathway. Genetics 2013, 193, 383–410. [Google Scholar] [CrossRef]
- Miller, E.A.; Beilharz, T.H.; Malkus, P.N.; Lee, M.C.; Hamamoto, S.; Orci, L.; Schekman, R. Multiple cargo binding sites on the copii subunit sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell 2003, 114, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.; Barlowe, C. Transport of axl2p depends on erv14p, an er-vesicle protein related to the drosophila cornichon gene product. J. Cell Biol. 1998, 142, 1209–1222. [Google Scholar] [CrossRef]
- Bue, C.A.; Bentivoglio, C.M.; Barlowe, C. Erv26p directs pro-alkaline phosphatase into endoplasmic reticulum-derived coat protein complex ii transport vesicles. Mol. Biol. Cell 2006, 17, 4780–4789. [Google Scholar] [CrossRef] [PubMed]
- Muniz, M.; Morsomme, P.; Riezman, H. Protein sorting upon exit from the endoplasmic reticulum. Cell 2001, 104, 313–320. [Google Scholar] [CrossRef]
- Belden, W.J.; Barlowe, C. Distinct roles for the cytoplasmic tail sequences of emp24p and erv25p in transport between the endoplasmic reticulum and golgi complex. J. Biol. Chem. 2001, 276, 43040–43048. [Google Scholar] [CrossRef] [PubMed]
- Castillon, G.A.; Watanabe, R.; Taylor, M.; Schwabe, T.M.; Riezman, H. Concentration of gpi-anchored proteins upon er exit in yeast. Traffic 2009, 10, 186–200. [Google Scholar] [CrossRef]
- Belden, W.J.; Barlowe, C. Role of erv29p in collecting soluble secretory proteins into er-derived transport vesicles. Science 2001, 294, 1528–1531. [Google Scholar] [CrossRef] [PubMed]
- Letourneur, F.; Gaynor, E.C.; Hennecke, S.; Demolliere, C.; Duden, R.; Emr, S.D.; Riezman, H.; Cosson, P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell 1994, 79, 1199–1207. [Google Scholar] [CrossRef]
- Semenza, J.C.; Hardwick, K.G.; Dean, N.; Pelham, H.R. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell 1990, 61, 1349–1357. [Google Scholar] [CrossRef]
- Nishikawa, S.; Nakano, A. Identification of a gene required for membrane protein retention in the early secretory pathway. Proc. Natl. Acad. Sci. USA 1993, 90, 8179–8183. [Google Scholar] [CrossRef] [PubMed]
- Andag, U.; Neumann, T.; Schmitt, H.D. The coatomer-interacting protein dsl1p is required for golgi-to-endoplasmic reticulum retrieval in yeast. J. Biol. Chem. 2001, 276, 39150–39160. [Google Scholar] [CrossRef] [PubMed]
- Meiringer, C.T.; Rethmeier, R.; Auffarth, K.; Wilson, J.; Perz, A.; Barlowe, C.; Schmitt, H.D.; Ungermann, C. The dsl1 protein tethering complex is a resident endoplasmic reticulum complex, which interacts with five soluble nsf (n-ethylmaleimide-sensitive factor) attachment protein receptors (snares): Implications for fusion and fusion regulation. J. Biol. Chem. 2011, 286, 25039–25046. [Google Scholar] [CrossRef] [PubMed]
- Losev, E.; Reinke, C.A.; Jellen, J.; Strongin, D.E.; Bevis, B.J.; Glick, B.S. Golgi maturation visualized in living yeast. Nature 2006, 441, 1002–1006. [Google Scholar] [CrossRef]
- Matsuura-Tokita, K.; Takeuchi, M.; Ichihara, A.; Mikuriya, K.; Nakano, A. Live imaging of yeast golgi cisternal maturation. Nature 2006, 441, 1007–1010. [Google Scholar] [CrossRef]
- Emr, S.; Glick, B.S.; Linstedt, A.D.; Lippincott-Schwartz, J.; Luini, A.; Malhotra, V.; Marsh, B.J.; Nakano, A.; Pfeffer, S.R.; Rabouille, C.; et al. Journeys through the golgi—taking stock in a new era. J. Cell Biol. 2009, 187, 449–453. [Google Scholar]
- Caro, L.G.; Palade, G.E. Protein synthesis, storage, and discharge in the pancreatic exocrine cell. An autoradiographic study. J. Cell Biol. 1964, 20, 473–495. [Google Scholar]
- Novick, P.; Ferro, S.; Schekman, R. Order of events in the yeast secretory pathway. Cell 1981, 25, 461–469. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef]
- TerBush, D.R.; Maurice, T.; Roth, D.; Novick, P. The exocyst is a multiprotein complex required for exocytosis in saccharomyces cerevisiae. EMBO J. 1996, 15, 6483–6494. [Google Scholar] [PubMed]
- Guo, W.; Roth, D.; Walch-Solimena, C.; Novick, P. The exocyst is an effector for sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999, 18, 1071–1080. [Google Scholar]
- Harsay, E.; Bretscher, A. Parallel secretory pathways to the cell surface in yeast. J. Cell Biol. 1995, 131, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Harsay, E.; Schekman, R. A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway. J. Cell Biol. 2002, 156, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.; Stevens, T.H. Protein transport from the late golgi to the vacuole in the yeast saccharomyces cerevisiae. Biochim. Biophys. Acta 2005, 1744, 438–454. [Google Scholar] [CrossRef]
- Raymond, C.K.; Howald-Stevenson, I.; Vater, C.A.; Stevens, T.H. Morphological classification of the yeast vacuolar protein sorting mutants: Evidence for a prevacuolar compartment in class E vps mutants. Mol. Biol. Cell 1992, 3, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.L.; Finlay, J.A.; Chu, D.S.; Tan, P.K.; Kirchhausen, T.; Payne, G.S. The saccharomyces cerevisiae APS1 gene encodes a homolog of the small subunit of the mammalian clathrin AP-1 complex: Evidence for functional interaction with clathrin at the Golgi complex. EMBO J. 1994, 13, 1706–1717. [Google Scholar] [PubMed]
- Black, M.W.; Pelham, H.R. A selective transport route from golgi to late endosomes that requires the yeast gga proteins. J. Cell Biol. 2000, 151, 587–600. [Google Scholar] [CrossRef]
- Boman, A.L.; Zhang, C.; Zhu, X.; Kahn, R.A. A family of adp-ribosylation factor effectors that can alter membrane transport through the trans-golgi. Mol. Biol. Cell 2000, 11, 1241–1255. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.; Lui, W.W.; Bright, N.A.; Totty, N.; Seaman, M.N.; Robinson, M.S. A family of proteins with γ-adaptin and vhs domains that facilitate trafficking between the trans-golgi network and the vacuole/lysosome. J. Cell Biol. 2000, 149, 67–80. [Google Scholar] [CrossRef]
- Duncan, M.C.; Costaguta, G.; Payne, G.S. Yeast epsin-related proteins required for golgi-endosome traffic define a γ-adaptin ear-binding motif. Nat. Cell Biol. 2003, 5, 77–81. [Google Scholar]
- Friant, S.; Pecheur, E.I.; Eugster, A.; Michel, F.; Lefkir, Y.; Nourrisson, D.; Letourneur, F. Ent3p is a ptdins(3,5)p2 effector required for protein sorting to the multivesicular body. Dev. Cell 2003, 5, 499–511. [Google Scholar] [CrossRef]
- Peplowska, K.; Markgraf, D.F.; Ostrowicz, C.W.; Bange, G.; Ungermann, C. The corvet tethering complex interacts with the yeast rab5 homolog vps21 and is involved in endo-lysosomal biogenesis. Dev. Cell 2007, 12, 739–750. [Google Scholar] [CrossRef]
- Wickner, W. Membrane fusion: Five lipids, four snares, three chaperones, two nucleotides, and a rab, all dancing in a ring on yeast vacuoles. Annu. Rev. Cell Dev. Biol. 2010, 26, 115–136. [Google Scholar] [CrossRef]
- Seaman, M.N.; McCaffery, J.M.; Emr, S.D. A membrane coat complex essential for endosome-to-golgi retrograde transport in yeast. J. Cell Biol. 1998, 142, 665–681. [Google Scholar] [CrossRef]
- Lauwers, E.; Erpapazoglou, Z.; Haguenauer-Tsapis, R.; Andre, B. The ubiquitin code of yeast permease trafficking. Trends Cell Biol. 2010, 20, 196–204. [Google Scholar] [CrossRef]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The escrt pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Katzmann, D.J.; Stefan, C.J.; Babst, M.; Emr, S.D. Vps27 recruits escrt machinery to endosomes during mvb sorting. J. Cell Biol. 2003, 162, 413–423. [Google Scholar] [CrossRef]
- Katzmann, D.J.; Babst, M.; Emr, S.D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 2001, 106, 145–155. [Google Scholar] [CrossRef]
- Azmi, I.F.; Davies, B.A.; Xiao, J.; Babst, M.; Xu, Z.; Katzmann, D.J. Escrt-iii family members stimulate vps4 atpase activity directly or via vta1. Dev. Cell 2008, 14, 50–61. [Google Scholar] [CrossRef]
- Reggiori, F.; Pelham, H.R. Sorting of proteins into multivesicular bodies: Ubiquitin-dependent and -independent targeting. EMBO J. 2001, 20, 5176–5186. [Google Scholar] [CrossRef] [PubMed]
- Stawiecka-Mirota, M.; Pokrzywa, W.; Morvan, J.; Zoladek, T.; Haguenauer-Tsapis, R.; Urban-Grimal, D.; Morsomme, P. Targeting of sna3p to the endosomal pathway depends on its interaction with Rsp5p and multivesicular body sorting on its ubiquitylation. Traffic 2007, 8, 1280–1296. [Google Scholar] [CrossRef]
- Cowles, C.R.; Snyder, W.B.; Burd, C.G.; Emr, S.D. Novel golgi to vacuole delivery pathway in yeast: Identification of a sorting determinant and required transport component. EMBO J. 1997, 16, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Odorizzi, G.; Cowles, C.R.; Emr, S.D. The AP-3 complex: A coat of many colours. Trends Cell Biol. 1998, 8, 282–288. [Google Scholar] [CrossRef]
- Munn, A.L.; Riezman, H. Endocytosis is required for the growth of vacuolar H(+)-ATPase-defective yeast: Identification of six new END genes. J. Cell Biol. 1994, 127, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Munn, A.L.; Stevenson, B.J.; Geli, M.I.; Riezman, H. End5, end6, and end7: Mutations that cause actin delocalization and block the internalization step of endocytosis in saccharomyces cerevisiae. Mol. Biol. Cell 1995, 6, 1721–1742. [Google Scholar] [CrossRef]
- Moreau, V.; Galan, J.M.; Devilliers, G.; Haguenauer-Tsapis, R.; Winsor, B. The yeast actin-related protein Arp2p is required for the internalization step of endocytosis. Mol. Biol. Cell 1997, 8, 1361–1375. [Google Scholar] [CrossRef] [PubMed]
- Madania, A.; Dumoulin, P.; Grava, S.; Kitamoto, H.; Scharer-Brodbeck, C.; Soulard, A.; Moreau, V.; Winsor, B. The saccharomyces cerevisiae homologue of human wiskott-aldrich syndrome protein las17p interacts with the arp2/3 complex. Mol. Biol. Cell 1999, 10, 3521–3538. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Toret, C.P.; Drubin, D.G. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2006, 7, 404–414. [Google Scholar] [CrossRef]
- Idrissi, F.Z.; Grotsch, H.; Fernandez-Golbano, I.M.; Presciatto-Baschong, C.; Riezman, H.; Geli, M.I. Distinct acto/myosin-I structures associate with endocytic profiles at the plasma membrane. J. Cell Biol. 2008, 180, 1219–1232. [Google Scholar]
- Idrissi, F.Z.; Geli, M.I. Zooming in on the molecular mechanisms of endocytic budding by time-resolved electron microscopy. Cell Mol. Life Sci. 2014, 71, 641–657. [Google Scholar] [CrossRef]
- Wiederkehr, A.; Avaro, S.; Prescianotto-Baschong, C.; Haguenauer-Tsapis, R.; Riezman, H. The f-box protein rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in saccharomyces cerevisiae. J. Cell Biol. 2000, 149, 397–410. [Google Scholar] [CrossRef]
- Furuta, N.; Fujimura-Kamada, K.; Saito, K.; Yamamoto, T.; Tanaka, K. Endocytic recycling in yeast is regulated by putative phospholipid translocases and the Ypt31p/32p-Rcy1p pathway. Mol. Biol. Cell 2007, 18, 295–312. [Google Scholar]
- Ziman, M.; Chuang, J.S.; Tsung, M.; Hamamoto, S.; Schekman, R. Chs6p-dependent anterograde transport of Chs3p from the chitosome to the plasma membrane in saccharomyces cerevisiae. Mol. Biol. Cell 1998, 9, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Bartnicki-Garcia, S. Chitosomes: Past, present and future. FEMS Yeast Res. 2006, 6, 957–965. [Google Scholar] [CrossRef]
- Valdivia, R.H.; Baggott, D.; Chuang, J.S.; Schekman, R.W. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late golgi membrane proteins. Dev. Cell 2002, 2, 283–294. [Google Scholar] [CrossRef]
- Copic, A.; Starr, T.L.; Schekman, R. Ent3p and Ent5p exhibit cargo-specific functions in trafficking proteins between the trans-golgi network and the endosomes in yeast. Mol. Biol. Cell 2007, 18, 1803–1815. [Google Scholar] [CrossRef]
- Copeland, D.E.; Dalton, A.J. An association between mitochondria and the endoplasmic reticulum in cells of the pseudobranch gland of a teleost. J. Biophys. Biochem. Cytol. 1959, 5, 393–396. [Google Scholar]
- Daum, G.; Vance, J.E. Import of lipids into mitochondria. Prog. Lipid Res. 1997, 36, 103–130. [Google Scholar] [CrossRef]
- Kornmann, B.; Currie, E.; Collins, S.R.; Schuldiner, M.; Nunnari, J.; Weissman, J.S.; Walter, P. An Er-mitochondria tethering complex revealed by a synthetic biology screen. Science 2009, 325, 477–481. [Google Scholar] [CrossRef]
- Voeltz, G.K.; Rolls, M.M.; Rapoport, T.A. Structural organization of the endoplasmic reticulum. EMBO Rep. 2002, 3, 944–950. [Google Scholar] [CrossRef]
- Honscher, C.; Ungermann, C. A close-up view of membrane contact sites between the endoplasmic reticulum and the endolysosomal system: From yeast to man. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 262–268. [Google Scholar] [CrossRef]
- Roberts, P.; Moshitch-Moshkovitz, S.; Kvam, E.; O’Toole, E.; Winey, M.; Goldfarb, D.S. Piecemeal microautophagy of nucleus in saccharomyces cerevisiae. Mol. Biol. Cell 2003, 14, 129–141. [Google Scholar]
- Elbaz-Alon, Y.; Rosenfeld-Gur, E.; Shinder, V.; Futerman, A.H.; Geiger, T.; Schuldiner, M. A dynamic interface between vacuoles and mitochondria in yeast. Dev. Cell 2014, 30, 95–102. [Google Scholar] [CrossRef]
- Honscher, C.; Mari, M.; Auffarth, K.; Bohnert, M.; Griffith, J.; Geerts, W.; van der Laan, M.; Cabrera, M.; Reggiori, F.; Ungermann, C. Cellular metabolism regulates contact sites between vacuoles and mitochondria. Dev. Cell 2014, 30, 86–94. [Google Scholar] [CrossRef]
- Klecker, T.; Bockler, S.; Westermann, B. Making connections: Interorganelle contacts orchestrate mitochondrial behavior. Trends Cell Biol. 2014, 24, 537–545. [Google Scholar] [CrossRef]
- Amoasii, L.; Bertazzi, D.L.; Tronchere, H.; Hnia, K.; Chicanne, G.; Rinaldi, B.; Cowling, B.S.; Ferry, A.; Klaholz, B.; Payrastre, B.; et al. Phosphatase-dead myotubularin ameliorates x-linked centronuclear myopathy phenotypes in mice. PLoS Genet. 2012, 8, e1002965. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feyder, S.; De Craene, J.-O.; Bär, S.; Bertazzi, D.L.; Friant, S. Membrane Trafficking in the Yeast Saccharomyces cerevisiae Model. Int. J. Mol. Sci. 2015, 16, 1509-1525. https://doi.org/10.3390/ijms16011509
Feyder S, De Craene J-O, Bär S, Bertazzi DL, Friant S. Membrane Trafficking in the Yeast Saccharomyces cerevisiae Model. International Journal of Molecular Sciences. 2015; 16(1):1509-1525. https://doi.org/10.3390/ijms16011509
Chicago/Turabian StyleFeyder, Serge, Johan-Owen De Craene, Séverine Bär, Dimitri L. Bertazzi, and Sylvie Friant. 2015. "Membrane Trafficking in the Yeast Saccharomyces cerevisiae Model" International Journal of Molecular Sciences 16, no. 1: 1509-1525. https://doi.org/10.3390/ijms16011509
APA StyleFeyder, S., De Craene, J. -O., Bär, S., Bertazzi, D. L., & Friant, S. (2015). Membrane Trafficking in the Yeast Saccharomyces cerevisiae Model. International Journal of Molecular Sciences, 16(1), 1509-1525. https://doi.org/10.3390/ijms16011509