Topical PDT in the Treatment of Benign Skin Diseases: Principles and New Applications
Abstract
:1. Introduction
2. Mechanism of Action of Photodynamic Therapy (PDT)
3. PDT Components
3.1. Photosensitizer
3.1.1. 5-Aminolevulinic Acid (ALA)-Induced Protoporphyrin IX (PPIX)
3.1.2. ALA-Ester-Induced PPIX
3.1.3. Other Topical Photosensitizers
3.2. Light Sources for Topical PDT

4. Therapeutic Applications of PDT in Benign Skin Diseases
4.1. PDT for Acne Vulgaris
| First Author, Year [Reference] | Type of Acne and Location | Number of Patients | Photosensitizer (Contact Time)/Light Source | Number of Treatment Sessions (Follow-up Time) | Clinical Results |
|---|---|---|---|---|---|
| Hongcharu, 2000 [55] | Inflammatory, mild to moderate/back | 22 | 20% ALA (3 h)/red light (550–570 nm) vs. red light only vs. placebo | Two randomized groups: 1 vs. 4 sessions (20 weeks) | ALA-PDT 1 session better than red light alone; After 20 weeks, 50% reduction of lesions after 4 sessions vs. ~30% reduction with 1 session |
| Itoh, 2001 [56] | Comedonal or inflammatory/face | 13 | 20% ALA (4 h)/broad-spectrum (600–700 nm) halogen lamp | 1 (24 weeks) | After 1 month, 100% some improvement without new lesions; at 3 months, 38.4% “excellent” response without new lesions |
| Goldman, 2003 [52] | Inflammatory, mild to moderate/face | 22 | 20% ALA (15 min)/blue light (417 ± 5 nm) vs. blue light only | 2 (2 weeks) | Reductions in inflammatory lesions: 68% with ALA-PDT vs. 40% with blue light only |
| Hong, 2005 [57] | Inflammatory, mild to moderate/face | 8 | 20% ALA (4 h)/halogen lamp red (630 ± 63 nm) | 1 (24 months) | Reductions in inflammatory lesions: 41.9% in treated sites vs. 15.4% in control |
| Wiegell, 2006 [58] | Inflammatory/Face | 15 | 20% ALA vs. 16.8% MAL (3 h)/noncoherent red (630 nm) | 1 (12 weeks) | Reductions in inflammatory lesions: 59% with both ALA and MAL; no significant difference between MAL and ALA sites |
| Rojanamatin, 2006 [59] | Inflammatory/face | 14 | 20% ALA (30 min)/IPL (cutoff filter, 560–590 nm) | 3 (12 weeks) | Reductions in inflammatory lesions: 87.7% for ALA-IPL vs. 66.8% for IPL only; not significantly different |
| Yeung, 2007 [60] | Inflammatory/face | 23 | 16.8% MAL (30 min)/IPL (530–750 nm) | 4 (12 weeks) | Reductions in inflammatory lesions: 65% with MAL-PDT vs. 23% with IPL only; noninflammatory lesions: 38% vs. 44% |
| Kim, 2009 [32] | Mild to moderate/face | 16 | 0.06% ICG solution (30 min)/near-infrared diode laser (805 nm) | 1 vs. 3 (8 weeks) | Subjective satisfaction score significantly higher in multiple-treatment group compared with a single-treatment group |
| Jang, 2011 [38] | Mild to moderate/face | 34 | IAA (30 min) with green light (520 nm) vs. ICG (15 min) with near-infrared radiation (805 nm) | 5 (3 months) | Reductions in inflammatory and noninflammatory lesions and sebum secretion: significant reductions for both IAA and ICG; no significant differences between IAA and ICG |
| Kim, 2012 [34] | Mild to moderate/face | 4 | 19% a,b-chlorophyll solution (30–60 min)/IPL (530–750 nm) | 3 (4 weeks) | All subjects: mild improvement after three sessions; significant reduction in lesion count at 1-month follow-up |
| Kwon, 2013 [54] | Mild to moderate/face | 55 | None/home use, combination blue–red LED (660 and 420 nm) vs. control (sham device) | Twice daily for 4 weeks (12 weeks) | At 12 weeks, reductions in both inflammatory and noninflammatory acne lesions |
| Yin, 2014 [61] | Inflammatory, moderate to severe/face | 40 | 15% ALA/ablative fractional Er:YAG laser + red light (633 ± 6 nm)/2 h | PDT: 4; Er:YAG laser: 5 (12 months) | After 6 months, 100% overall improvement in inflammatory lesions; 80% overall improvement in acne scars without recurrence |

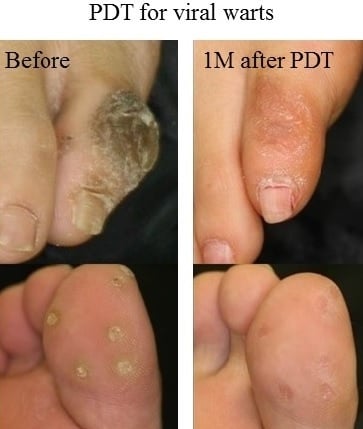
4.2. PDT for Refractory Palmoplantar Warts

4.3. PDT for Genital Warts
4.4. PDT for Photorejuvenation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rabb, O. Ueber die wirkung fluoreszierender stoffe auf infusori. Ztg. Biol. 1900, 39, 524–536. [Google Scholar]
- Von Tappeiner, H.; Jesionek, A. Therapeutische versuche mit fluoreszierenden stoffen. Munch. Med. Wochenschr. 1903, 50, 2042–2044. [Google Scholar]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar] [PubMed]
- Jin, Y.; Zhang, X.; Zhang, B.; Kang, H.; Du, L.; Li, M. Nanostructures of an amphiphilic zinc phthalocyanine polymer conjugate for photodynamic therapy of psoriasis. Colloids Surf. B Biointerfaces 2015, 128, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Tremblay, J.F.; Juzenas, P.; Boushira, M.; Lui, H. Systemic photodynamic therapy with aminolevulinic acid induces apoptosis in lesional T lymphocytes of psoriatic plaques. J. Investig. Dermatol. 2002, 119, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Karrer, S.; Abels, C.; Wimmershoff, M.B.; Landthaler, M.; Szeimies, R.M. Successful treatment of cutaneous sarcoidosis using topical photodynamic therapy. Arch. Dermatol. 2002, 138, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, Y.; Wang, Q.; Ren, J.; Xiang, L. Prospective study of topical 5-aminolevulinic acid photodynamic therapy for the treatment of severe adolescent acne in Chinese patients. J. Dermatol. 2015, 42, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Li, X.; Dang, H. 5-aminolevulinic acid-based photodynamic therapy for the treatment of condylomata acuminata in Chinese patients: A meta-analysis. Photodermatol. Photoimmunol. Photomed. 2013, 29, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Enk, C.D.; Nasereddin, A.; Alper, R.; Dan-Goor, M.; Jaffe, C.L.; Wulf, H.C. Cutaneous leishmaniasis responds to daylight-activated photodynamic therapy: Proof of concept for a novel self-administered therapeutic modality. Br. J. Dermatol. 2015, 172, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef]
- Morton, C.A.; Szeimies, R.M.; Sidoroff, A.; Braathen, L.R. European guidelines for topical photodynamic therapy part 1: Treatment delivery and current indications—Actinic keratoses, Bowen’s disease, basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell death pathways in photodynamic therapy of cancer. Cancers (Basel) 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [PubMed]
- Karrer, S.; Bosserhoff, A.K.; Weiderer, P.; Landthaler, M.; Szeimies, R.M. Keratinocyte-derived cytokines after photodynamic therapy and their paracrine induction of matrix metalloproteinases in fibroblasts. Br. J. Dermatol. 2004, 151, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.C.; Pottier, R.H.; Pross, D.C. Photodynamic therapy with endogenous protoporphyrin IX: Basic principles and present clinical experience. J. Photochem. Photobiol. B Biol. 1990, 6, 143–148. [Google Scholar] [CrossRef]
- Iinuma, S.; Farshi, S.S.; Ortel, B.; Hasan, T. A mechanistic study of cellular photodestruction with 5-aminolaevulinic acid-induced porphyrin. Br. J. Cancer 1994, 70, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Divaris, D.X.; Kennedy, J.C.; Pottier, R.H. Phototoxic damage to sebaceous glands and hair follicles of mice after systemic administration of 5-aminolevulinic acid correlates with localized protoporphyrin IX fluorescence. Am. J. Pathol. 1990, 136, 891–897. [Google Scholar] [PubMed]
- Szeimies, R.M.; Landthaler, M. Photodynamic therapy and fluorescence diagnosis of skin cancers. Recent Results Cancer Res. 2002, 160, 240–245. [Google Scholar] [PubMed]
- Barr, H.; Kendall, C.; Reyes-Goddard, J.; Stone, N. Clinical aspects of photodynamic therapy. Sci. Prog. 2002, 85, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; Perotti, C.; Saccoliti, M.; Sacca, P.; Fukuda, H.; Batlle, A.M. ALA and ALA hexyl ester in free and liposomal formulations for the photosensitisation of tumour organ cultures. Br. J. Cancer 2002, 86, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Di Venosa, G.; Hermida, L.; Batlle, A.; Fukuda, H.; Defain, M.V.; Mamone, L.; Rodriguez, L.; MacRobert, A.; Casas, A. Characterisation of liposomes containing aminolevulinic acid and derived esters. J. Photochem. Photobiol. B Biol. 2008, 92, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sadasivam, M.; Avci, P.; Gupta, G.K.; Lakshmanan, S.; Chandran, R.; Huang, Y.Y.; Kumar, R.; Hamblin, M.R. Self-assembled liposomal nanoparticles in photodynamic therapy. Eur. J. Nanomed. 2013, 5, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Dirschka, T.; Radny, P.; Dominicus, R.; Mensing, H.; Bruning, H.; Jenne, L.; Karl, L.; Sebastian, M.; Oster-Schmidt, C.; Klovekorn, W.; et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: Results of a multicentre, randomized, observer-blind phase III study in comparison with a registered methyl-5-aminolaevulinate cream and placebo. Br. J. Dermatol. 2012, 166, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Weingandt, H.; Liessmann, F.; Klein, S.; Stepp, H.; Baumgartner, R.; Hillemanns, P. Photodynamic effects induced by aminolevulinic acid esters on human cervical carcinoma cells in culture. Photochem. Photobiol. 2001, 74, 617–623. [Google Scholar] [CrossRef]
- Braathen, L.R.; Szeimies, R.M.; Basset-Seguin, N.; Bissonnette, R.; Foley, P.; Pariser, D.; Roelandts, R.; Wennberg, A.M.; Morton, C.A. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: An international consensus. International Society for Photodynamic Therapy in Dermatology, 2005. J. Am. Acad. Dermatol. 2007, 56, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Szeimies, R.M.; Karrer, S.; Radakovic-Fijan, S.; Tanew, A.; Calzavara-Pinton, P.G.; Zane, C.; Sidoroff, A.; Hempel, M.; Ulrich, J.; Proebstle, T.; et al. Photodynamic therapy using topical methyl 5-aminolevulinate compared with cryotherapy for actinic keratosis: A prospective, randomized study. J. Am. Acad. Dermatol. 2002, 47, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Pariser, D.M.; Lowe, N.J.; Stewart, D.M.; Jarratt, M.T.; Lucky, A.W.; Pariser, R.J.; Yamauchi, P.S. Photodynamic therapy with topical methyl aminolevulinate for actinic keratosis: Results of a prospective randomized multicenter trial. J. Am. Acad. Dermatol. 2003, 48, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Tarstedt, M.; Rosdahl, I.; Berne, B.; Svanberg, K.; Wennberg, A.M. A randomized multicenter study to compare two treatment regimens of topical methyl aminolevulinate (Metvix)-PDT in actinic keratosis of the face and scalp. Acta Derm. Venereol. 2005, 85, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, D.I.; Thissen, M.R.; Thissen, C.A.; Neumann, M.H. Similar effectiveness of methyl aminolevulinate and 5-aminolevulinate in topical photodynamic therapy for nodular basal cell carcinoma. J. Drugs Dermatol. 2006, 5, 642–645. [Google Scholar] [PubMed]
- Moloney, F.J.; Collins, P. Randomized, double-blind, prospective study to compare topical 5-aminolaevulinic acid methylester with topical 5-aminolaevulinic acid photodynamic therapy for extensive scalp actinic keratosis. Br. J. Dermatol. 2007, 157, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Botto, N.; Rogers, G. Nontraditional management of basal cell carcinoma. J. Drugs Dermatol. 2013, 12, 525–532. [Google Scholar] [PubMed]
- Fritsch, C.; Homey, B.; Stahl, W.; Lehmann, P.; Ruzicka, T.; Sies, H. Preferential relative porphyrin enrichment in solar keratoses upon topical application of delta-aminolevulinic acid methylester. Photochem. Photobiol. 1998, 68, 218–221. [Google Scholar] [PubMed]
- Kim, B.J.; Lee, H.G.; Woo, S.M.; Youn, J.I.; Suh, D.H. Pilot study on photodynamic therapy for acne using indocyanine green and diode laser. J. Dermatol. 2009, 36, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Barras, A.; Boussekey, L.; Courtade, E.; Boukherroub, R. Hypericin-loaded lipid nanocapsules for photodynamic cancer therapy in vitro. Nanoscale 2013, 5, 10562–10572. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Hwang, J.I.; Lee, J.I.; Cho, B.K.; Park, H.J. Pilot study on photodynamic therapy for acne using chlorophyll: Evaluator-blinded, split-face study. J. Dermatol. Treat. 2012, 23, 35–36. [Google Scholar] [CrossRef] [PubMed]
- Paz-Cristobal, M.P.; Gilaberte, Y.; Alejandre, C.; Pardo, J.; Revillo, M.J.; Rezusta, A. In vitro fungicidal photodynamic effect of hypericin on Trichophyton spp. Mycopathologia 2014, 178, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T. Recent overview of the Mg branch of the tetrapyrrole biosynthesis leading to chlorophylls. Photosynth. Res. 2008, 96, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Nyman, E.S.; Hynninen, P.H. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2004, 73, 1–28. [Google Scholar] [CrossRef]
- Jang, M.S.; Doh, K.S.; Kang, J.S.; Jeon, Y.S.; Suh, K.S.; Kim, S.T. A comparative split-face study of photodynamic therapy with indocyanine green and indole-3-acetic acid for the treatment of acne vulgaris. Br. J. Dermatol. 2011, 165, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.H. Therapeutic and aesthetic uses of photodynamic therapy part two of a five-part series: Lasers and light treatments for acne vulgaris promising therapies. J. Clin. Aesthet. Dermatol. 2008, 1, 28–34. [Google Scholar] [PubMed]
- Karrer, S.; Baumler, W.; Abels, C.; Hohenleutner, U.; Landthaler, M.; Szeimies, R.M. Long-pulse dye laser for photodynamic therapy: Investigations in vitro and in vivo. Lasers Surg. Med. 1999, 25, 51–59. [Google Scholar] [CrossRef]
- Itkin, A.; Gilchrest, B.A. delta-Aminolevulinic acid and blue light photodynamic therapy for treatment of multiple basal cell carcinomas in two patients with nevoid basal cell carcinoma syndrome. Dermatol. Surg. 2004, 30, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Barge, J.; Glanzmann, T.; Zellweger, M.; Salomon, D.; van den Bergh, H.; Wagnieres, G. Correlations between photoactivable porphyrins’ fluorescence, erythema and the pain induced by PDT on normal skin using ALA-derivatives. Photodiagnosis Photodyn. Ther. 2013, 10, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Piffaretti, F.; Zellweger, M.; Kasraee, B.; Barge, J.; Salomon, D.; van den Bergh, H.; Wagnieres, G. Correlation between protoporphyrin IX fluorescence intensity, photobleaching, pain and clinical outcome of actinic keratosis treated by photodynamic therapy. Dermatology (Basel) 2013, 227, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Borelli, C.; Merk, K.; Schaller, M.; Jacob, K.; Vogeser, M.; Weindl, G.; Berger, U.; Plewig, G. In vivo porphyrin production by P. acnes in untreated acne patients and its modulation by acne treatment. Acta Derm. Venereol. 2006, 86, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, H.; Malik, Z.; Harth, Y.; Nitzan, Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol. Med. Microbiol. 2003, 35, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Titus, S.; Hodge, J. Diagnosis and treatment of acne. Am. Fam. Physician 2012, 86, 734–740. [Google Scholar] [PubMed]
- Thiboutot, D.; Gollnick, H.; Bettoli, V.; Dreno, B.; Kang, S.; Leyden, J.J.; Shalita, A.R.; Lozada, V.T.; Berson, D.; Finlay, A.; et al. New insights into the management of acne: An update from the global alliance to improve outcomes in acne group. J. Am. Acad. Dermatol. 2009, 60, S1–S50. [Google Scholar] [CrossRef] [PubMed]
- James, W.D. Clinical practice. Acne. N. Engl. J. Med. 2005, 352, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Ninomiya, Y.; Tajima, S.; Ishibashi, A. Photodynamic therapy for acne vulgaris with topical 5-aminolevulinic acid. Arch. Dermatol. 2000, 136, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, A.; Kjeldstad, B.; Melo, T.B. Fluorescence from pilosebaceous follicles. Arch. Dermatol. Res. 1987, 279, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, W.J.; Goulden, V. Phototherapy and acne vulgaris. Br. J. Dermatol. 2000, 142, 855–856. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.P.; Boyce, S.M. A single-center study of aminolevulinic acid and 417 NM photodynamic therapy in the treatment of moderate to severe acne vulgaris. J. Drugs Dermatol. 2003, 2, 393–396. [Google Scholar] [PubMed]
- Kawada, A.; Aragane, Y.; Kameyama, H.; Sangen, Y.; Tezuka, T. Acne phototherapy with a high-intensity, enhanced, narrow-band, blue light source: An open study and in vitro investigation. J. Dermatol. Sci. 2002, 30, 129–135. [Google Scholar] [CrossRef]
- Kwon, H.H.; Lee, J.B.; Yoon, J.Y.; Park, S.Y.; Ryu, H.H.; Park, B.M.; Kim, Y.J.; Suh, D.H. The clinical and histological effect of home-use, combination blue–red LED phototherapy for mild-to-moderate acne vulgaris in Korean patients: A double-blind, randomized controlled trial. Br. J. Dermatol. 2013, 168, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Hongcharu, W.; Taylor, C.R.; Chang, Y.; Aghassi, D.; Suthamjariya, K.; Anderson, R.R. Topical ALA-photodynamic therapy for the treatment of acne vulgaris. J. Investig. Dermatol. 2000, 115, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Ninomiya, Y.; Tajima, S.; Ishibashi, A. Photodynamic therapy of acne vulgaris with topical δ-aminolaevulinic acid and incoherent light in Japanese patients. Br. J. Dermatol. 2001, 144, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.B.; Lee, M.H. Topical aminolevulinic acid-photodynamic therapy for the treatment of acne vulgaris. Photodermatol. Photoimmunol. Photomed. 2005, 21, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Wiegell, S.R.; Wulf, H.C. Photodynamic therapy of acne vulgaris using 5-aminolevulinic acid versus methyl aminolevulinate. J. Am. Acad. Dermatol. 2006, 54, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Rojanamatin, J.; Choawawanich, P. Treatment of inflammatory facial acne vulgaris with intense pulsed light and short contact of topical 5-aminolevulinic acid: A pilot study. Dermatol. Surg. 2006, 32, 991–996. [Google Scholar] [PubMed]
- Yeung, C.K.; Shek, S.Y.; Bjerring, P.; Yu, C.S.; Kono, T.; Chan, H.H. A comparative study of intense pulsed light alone and its combination with photodynamic therapy for the treatment of facial acne in Asian skin. Lasers Surg. Med. 2007, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Lin, L.; Xiao, Y.; Hao, F.; Hamblin, M.R. Combination ALA-PDT and ablative fractional Er:YAG laser (2940 nm) on the treatment of severe acne. Lasers Surg. Med. 2014, 46, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, F.H.; Lopes, J.D.; Anderson, R.R. Photodynamic therapy for acne vulgaris: A critical review from basics to clinical practice: Part I. Acne vulgaris: When and why consider photodynamic therapy? J. Am. Acad. Dermatol. 2010, 63, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Wiegell, S.R.; Stender, I.M.; Na, R.; Wulf, H.C. Pain associated with photodynamic therapy using 5-aminolevulinic acid or 5-aminolevulinic acid methylester on tape-stripped normal skin. Arch. Dermatol. 2003, 139, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.T.; Lin, J.Y. Current evidence and applications of photodynamic therapy in dermatology. Clin. Cosmet. Investig. Dermatol. 2014, 7, 145–163. [Google Scholar] [PubMed]
- Stender, I.M.; Lock-Andersen, J.; Wulf, H.C. Recalcitrant hand and foot warts successfully treated with photodynamic therapy with topical 5-aminolaevulinic acid: A pilot study. Clin. Exp. Dermatol. 1999, 24, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.H.; Pope, A. Fractional resurfacing aiding photodynamic therapy of a recalcitrant plantar verruca: A case report and review of the literature. J. Clin. Aesthet. Dermatol. 2008, 1, 30–33. [Google Scholar] [PubMed]
- Kim, J.E.; Kim, S.J.; Hwang, J.I.; Lee, K.J.; Park, H.J.; Cho, B.K. New proposal for the treatment of viral warts with intralesional injection of 5-aminolevulinic acid photodynamic therapy. J. Dermatol. Treat. 2012, 23, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Stender, I.M.; Na, R.; Fogh, H.; Gluud, C.; Wulf, H.C. Photodynamic therapy with 5-aminolaevulinic acid or placebo for recalcitrant foot and hand warts: Randomised double-blind trial. Lancet 2000, 355, 963–966. [Google Scholar] [CrossRef]
- Mizuki, D.; Kaneko, T.; Hanada, K. Successful treatment of topical photodynamic therapy using 5-aminolevulinic acid for plane warts. Br. J. Dermatol. 2003, 149, 1087–1088. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.G.; Wu, J.J.; He, Y.; Yang, H.Z.; Yang, Y.D. Efficacy of topical aminolevulinic acid photodynamic therapy for the treatment of verruca planae. Photomed. Laser Surg. 2010, 28, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, C.A.; Kaas, L.; Waterval, J.J.; Bos, P.M.; Neumann, H.A. Successful treatment of periungual warts using photodynamic therapy: A pilot study. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.S.; Kang, G.Y. Dramatic clearance of a recalcitrant acral viral wart using methyl aminolevulinate-red light photodynamic therapy. Photodermatol. Photoimmunol. Photomed. 2009, 25, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chang, B.Z.; Ju, M.; Zhang, X.H.; Gu, H. Comparative study of photodynamic therapy vs. CO2 laser vaporization in treatment of condylomata acuminata: A randomized clinical trial. Br. J. Dermatol. 2007, 156, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Fehr, M.K.; Chapman, C.F.; Krasieva, T.; Tromberg, B.J.; McCullough, J.L.; Berns, M.W.; Tadir, Y. Selective photosensitizer distribution in vulvar condyloma acuminatum after topical application of 5-aminolevulinic acid. Am. J. Obstet. Gynecol. 1996, 174, 951–957. [Google Scholar] [CrossRef]
- Ichimura, H.; Yamaguchi, S.; Kojima, A.; Tanaka, T.; Niiya, K.; Takemori, M.; Hasegawa, K.; Nishimura, R. Eradication and reinfection of human papillomavirus after photodynamic therapy for cervical intraepithelial neoplasia. Int. J. Clin. Oncol. 2003, 8, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Istomin, Y.P.; Lapzevich, T.P.; Chalau, V.N.; Shliakhtsin, S.V.; Trukhachova, T.V. Photodynamic therapy of cervical intraepithelial neoplasia grades II and III with Photolon. Photodiagnosis Photodyn. Ther. 2010, 7, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Nucci, V.; Torchia, D.; Cappugi, P. Treatment of anogenital condylomata acuminata with topical photodynamic therapy: Report of 14 cases and review. Int. J. Infect. Dis. 2010, 14 (Suppl. 3), e280–e282. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.V.; Romero, R.; Kollias, N.; Crum, C.; Anderson, R.R. Selectivity of protoporphyrin IX fluorescence for condylomata after topical application of 5-aminolaevulinic acid: Implications for photodynamic treatment. Br. J. Dermatol. 1997, 137, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; Chen, J.; He, Q.; Xiang, L.; Huang, X.; Ding, G.; Xu, S. Successful photodynamic therapy with topical 5-aminolevulinic acid for five cases of cervical intraepithelial neoplasia. Arch. Gynecol. Obstet. 2010, 282, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Wang, H.W.; Wang, H.S.; Xu, S.Z.; Liao, K.H.; Hillemanns, P. Topical 5-aminolaevulinic acid-photodynamic therapy for the treatment of urethral condylomata acuminata. Br. J. Dermatol. 2004, 151, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Lu, X.N.; Tang, H.; Zhang, Z.; Fan, J.; Xu, J.H. Evaluation of photodynamic therapy using topical aminolevulinic acid hydrochloride in the treatment of condylomata acuminata: A comparative, randomized clinical trial. Photodermatol. Photoimmunol. Photomed. 2009, 25, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Szeimies, R.M.; Schleyer, V.; Moll, I.; Stocker, M.; Landthaler, M.; Karrer, S. Adjuvant photodynamic therapy does not prevent recurrence of condylomata acuminata after carbon dioxide laser ablation-A phase III, prospective, randomized, bicentric, double-blind study. Dermatol. Surg. 2009, 35, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Zakhary, K.; Ellis, D.A. Applications of aminolevulinic Acid-based photodynamic therapy in cosmetic facial plastic practices. Facial Plast. Surg. 2005, 21, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Uebelhoer, N.S.; Dover, J.S. Photodynamic therapy for cosmetic applications. Dermatol. Ther. 2005, 18, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Babilas, P.; Travnik, R.; Werner, A.; Landthaler, M.; Szeimies, R.M. Split-face-study using two different light sources for topical PDT of actinic keratoses: Non-inferiority of the LED system. J. Dtsch. Dermatol. Ges. 2008, 6, 25–32. [Google Scholar] [PubMed]
- Gold, M.H.; Bradshaw, V.L.; Boring, M.M.; Bridges, T.M.; Biron, J.A. Split-face comparison of photodynamic therapy with 5-aminolevulinic acid and intense pulsed light versus intense pulsed light alone for photodamage. Dermatol. Surg. 2006, 32, 795–801. [Google Scholar] [PubMed]
- Shin, H.T.; Kim, J.H.; Shim, J.; Lee, J.H.; Lee, D.Y.; Lee, J.H.; Yang, J.M. Photodynamic therapy using a new formulation of 5-aminolevulinic acid for wrinkles in Asian skin: A randomized controlled split face study. J. Dermatolog. Treat. 2015, 26, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Kohl, E.; Torezan, L.A.; Landthaler, M.; Szeimies, R.M. Aesthetic effects of topical photodynamic therapy. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Marmur, E.S.; Phelps, R.; Goldberg, D.J. Ultrastructural changes seen after ALA-IPL photorejuvenation: A pilot study. J. Cosmet. Laser Ther. 2005, 7, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Orringer, J.S.; Hammerberg, C.; Hamilton, T.; Johnson, T.M.; Kang, S.; Sachs, D.L.; Fisher, G.; Voorhees, J.J. Molecular effects of photodynamic therapy for photoaging. Arch. Dermatol. 2008, 144, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Jung, H.Y.; Park, H.J. Topical PDT in the Treatment of Benign Skin Diseases: Principles and New Applications. Int. J. Mol. Sci. 2015, 16, 23259-23278. https://doi.org/10.3390/ijms161023259
Kim M, Jung HY, Park HJ. Topical PDT in the Treatment of Benign Skin Diseases: Principles and New Applications. International Journal of Molecular Sciences. 2015; 16(10):23259-23278. https://doi.org/10.3390/ijms161023259
Chicago/Turabian StyleKim, Miri, Haw Young Jung, and Hyun Jeong Park. 2015. "Topical PDT in the Treatment of Benign Skin Diseases: Principles and New Applications" International Journal of Molecular Sciences 16, no. 10: 23259-23278. https://doi.org/10.3390/ijms161023259
APA StyleKim, M., Jung, H. Y., & Park, H. J. (2015). Topical PDT in the Treatment of Benign Skin Diseases: Principles and New Applications. International Journal of Molecular Sciences, 16(10), 23259-23278. https://doi.org/10.3390/ijms161023259