Staphylococcus aureus and MRSA Growth and Biofilm Formation after Treatment with Antibiotics and SeNPs
Abstract
:1. Introduction
2. Results and Discussion
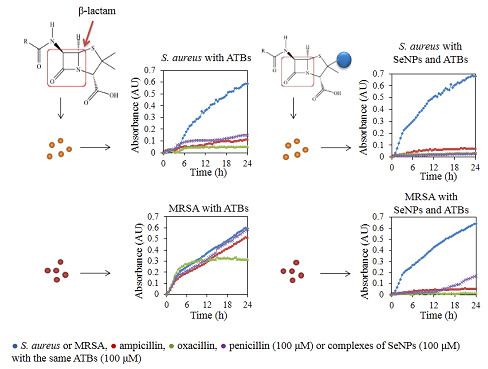
2.1. Influence of Antibacterial Compounds to Growth Properties

2.2. Influence of Antibacterial Compounds to Biofilm Formation

| Compounds | Biofilm Disruption (%) | |||
|---|---|---|---|---|
| S. aureus | MRSA | |||
| ATB | SeNPs + ATBs | ATB | SeNPs + ATBs | |
| AMP | 74 ± 2 | 93 ± 3 | 6 ± 5 | 94 ± 4 |
| OXA | 79 ± 5 | 96 ± 2 | 16 ± 2 | 93 ± 4 |
| PNC | 71 ± 2 | 99 ± 7 | 0 | 86 ± 2 |
| SeNPs | 81 ± 4 | 55 ± 3 | ||
2.3. Determination of Expression Intensity of mecA Gene

2.4. Determination of Changes in Protein Structure

| Strain | MIC (µM) | FICI | |||||
|---|---|---|---|---|---|---|---|
| AMP | OXA | PNC | SeNPs | SeNPs + AMP | SeNPs + OXA | SeNPs + PNC | |
| S. aureus | 50 | 25 | 50 | 10 | 0.70 | 0.90 | 0.70 |
| MRSA | 300 | 150 | 300 | 20 | 0.53 | 0.57 | 0.53 |
3. Experimental Section
3.1. Cultivation of S. aureus and MRSA
3.2. Testing of Antibacterial Properties
3.3. Preparation of the SeNPs and Complexes of SeNPs with ATBs
3.4. Measuring the Biofilm Formed by S. aureus and MRSA Followed by Application of ATBs
3.5. Gene Expression
3.5.1. Isolation of RNA
3.5.2. Reverse Transcription and Amplification of cDNA for mecA Gene
3.5.3. Visualization and Quantification of Gene Expression
3.6. Determination of Protein Fingerprints by MALDI-TOF
3.7. The Checkerboard Dilution Test
3.8. Descriptive Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ding, Y.L.; Wang, W.; Fan, M.; Tong, Z.C.; Kuang, R.; Jiang, W.K.; Ni, L.X. Antimicrobial and anti-biofilm effect of Bac8c on major bacteria associated with dental caries and Streptococcus mutans biofilms. Peptides 2014, 52, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Abucayon, E.; Ke, N.; Cornut, R.; Patelunas, A.; Miller, D.; Nishiguchi, M.K.; Zoski, C.G. Investigating catalase activity through hydrogen peroxide decomposition by bacteria biofilms in real time using scanning electrochemical microscopy. Anal. Chem. 2014, 86, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Chudobova, D.; Nejdl, L.; Gumulec, J.; Krystofova, O.; Rodrigo, M.A.M.; Kynicky, J.; Ruttkay-Nedecky, B.; Kopel, P.; Babula, P.; Adam, V.; et al. Complexes of silver(I) ions and silver phosphate nanoparticles with hyaluronic acid and/or chitosan as promising antimicrobial agents for vascular grafts. Int. J. Mol. Sci. 2013, 14, 13592–13614. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.T.R.; Al Omran, S.; Al Aithan, A.S.M.; Al Sultan, M. Efficacy and safety of low-dose colistin in the treatment for infections caused by multidrug-resistant gram-negative bacteria. J. Clin. Pharm. Ther. 2014, 39, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Buru, A.S.; Pichika, M.R.; Neela, V.; Mohandas, K. In vitro antibacterial effects of Cinnamomum extracts on common bacteria found in wound infections with emphasis on methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 2014, 153, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Ciofu, O.; Molin, S.; Givskov, M.; Hoiby, N. Applying insights from biofilm biology to drug development—Can a new approach be developed? Nat. Rev. Drug Discov. 2013, 12, 791–808. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; de Peralta, T.; Handy, R.D. Inhibition of biofilm formation and antibacterial properties of a silver nano-coating on human dentine. Nanotoxicology 2014, 8, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Banaszkiewicz, T.; Krukowski, H. Pathogenicity of MRSA for humans and animals. Med. Weter. 2014, 70, 151–156. [Google Scholar]
- Lall, M.; Sahni, A.K. Prevalence of inducible clindamycin resistance in Staphylococcus aureus isolated from clinical samples. Armed. Forces Med. J. India 2014, 70, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; He, N.A.; Cheng, T.; Tan, H.L.; Guo, Y.Y.; Chen, D.S.; Cheng, M.Q.; Yang, Z.; Zhang, X.L. Ultrasound-targeted microbubble destruction enhances human beta-defensin 3 activity against antibiotic-resistant Staphylococcus biofilms. Inflammation 2013, 36, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Mwangi, M.; Chung, M.; Milheirco, C.; de Lencastre, H.; Tomasz, A. The mechanism of heterogeneous beta-lactam resistance in MRSA: Key role of the stringent stress response. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vanderhaeghen, W.; Vandendriessche, S.; Crombe, F.; Dispas, M.; Denis, O.; Hermans, K.; Haesebrouck, F.; Butaye, P. Species and staphylococcal cassette chromosome mec (SCCmec) diversity among methicillin-resistant non-Staphylococcus aureus staphylococci isolated from pigs. Vet. Microbiol. 2012, 158, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-J.; Jia, B.; Wei, X.-Y.; Huang, W.-X.; Liu, C.-W. Tracing the outbreak of methicilin resistant Staphylococcus aureus in intensive care units. Zhongguo Kangshengsu Zazhi 2012, 37, 615–618. (In Chinese) [Google Scholar]
- Boneca, I.G.; Chiosis, G. Vancomycin resistance: Occurrence, mechanisms and strategies to combat it. Expert Opin. Ther. Targets 2003, 7, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Hibberd, P.L.; Goldin, B.; Thorpe, C.; McDermott, L.; Snydman, D.R. Effect of lactobacillus rhamnosus GG administration on vancomycin-resistant enterococcus colonization in adults with comorbidities. Antimicrob. Agents Chemther. 2015, 59, 4593–4599. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, K.; Okuma, K.; Ma, X.X.; Yamamoto, M.; Hori, S.; Kapi, M. New trends in Staphylococcus aureus infections: Glycopeptide resistance in hospital and methicillin resistance in the community. Curr. Opin. Infect. Dis. 2002, 15, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-spectrum bioactivities of silver nanoparticles: The emerging trends and future prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.A.; Fadl-allah, S.A.; El-Bagoury, N.; El-Rab, S. Improvement of corrosion resistance and antibacterial effect of NiTi orthopedic materials by chitosan and gold nanoparticles. Appl. Surf. Sci. 2014, 292, 390–399. [Google Scholar] [CrossRef]
- Kummer, K.M.; Taylor, E.N.; Durmas, N.G.; Tarquinio, K.M.; Ercan, B.; Webster, T.J. Effects of different sterilization techniques and varying anodized TiO2 nanotube dimensions on bacteria growth. J. Biomed. Mater. Res. Part B 2013, 101B, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Mirershadi, F.; Jafari, A.; Janati, E.; Roohi, E.; Sarabi, M. Ag/ZnO nano-composites as novel antibacterial agent against strain of MRSA. J. Pure Appl. Microbiol. 2013, 7, 947–956. [Google Scholar]
- Chudobova, D.; Cihalova, K.; Dostalova, S.; Ruttkay-Nedecky, B.; Rodrigo, M.A.M.; Tmejova, K.; Kopel, P.; Nejdl, L.; Kudr, J.; Gumulec, J.; et al. Comparison of the effects of silver phosphate and selenium nanoparticles on Staphylococcus aureus growth reveals potential for selenium particles to prevent infection. FEMS Microbiol. Lett. 2014, 351, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.A.; Webster, T.J. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomed. 2011, 6, 1553–1558. [Google Scholar]
- Singh, R.; Smitha, M.S.; Singh, S.P. The role of nanotechnology in combating multi-drug resistant bacteria. J. Nanosci. Nanotechnol. 2014, 14, 4745–4756. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.J.; Kim, H.S.; Jun, S.H.; Kang, Y.H.; Cho, S.; Park, Y. Biogenic silver nanoparticles with chlorogenic acid as a bioreducing agent. J. Nanosci. Nanotechnol. 2013, 13, 5787–5793. [Google Scholar] [CrossRef] [PubMed]
- Grigor’eva, A.; Saranina, I.; Tikunova, N.; Safonov, A.; Timoshenko, N.; Rebrov, A.; Ryabchikova, E. Fine mechanisms of the interaction of silver nanoparticles with the cells of Salmonella typhimurium and Staphylococcus aureus. Biometals 2013, 26, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.N.; Gutierrez, L.; McNulty, A. Real-time cellular analysis as a novel approach for in vitro cytotoxicity testing of medical device extracts. J. Biomed. Mater. Res. Part A 2013, 101A, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Scrace, S.; O’Neill, E.; Hammond, E.M.; Pires, I.M. Use of the xCELLigence system for real-time analysis of changes in cellular motility and adhesion in physiological conditions. In Adhesion Protein Protocols, 3rd ed.; Coutts, A.S., Ed.; Humana Press Inc: Totowa, UK, 2013; Volume 1046, pp. 295–306. [Google Scholar]
- Junka, A.F.; Janczura, A.; Smutnicka, D.; Maczynska, B.; Secewicz, A.; Nowicka, J.; Bartoszewicz, M.; Gosciniak, G. Use of the real time xCelligence system for purposes of medical microbiology. Pol. J. Microbiol. 2012, 61, 191–197. [Google Scholar]
- Wagner, C.; Aytac, S.; Hansch, G.M. Biofilm growth on implants: Bacteria prefer plasma coats. Int. J. Artif. Organs 2011, 34, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, P.; Mojica, K.D.A.; Allen, J.S.; Matter, M.L. Detection and quantification of bacterial biofilms combining high-frequency acoustic microscopy and targeted lipid microparticles. J. Nanobiotechnol. 2014, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Muhsin, T.M.; Hachim, A.K. Mycosynthesis and characterization of silver nanoparticles and their activity against some human pathogenic bacteria. World J. Microbiol. Biotechnol. 2014, 30, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Wang, X.-J.; Ma, J. Progress in real time xCELLigence analysis system on drug cardiotoxicity screening. Zhongguo Yaolixue Yu Dulixue Zazhi 2013, 27, 908–912. (In Chinese) [Google Scholar]
- Himmel, H.; Herbold, S. Drug-induced functional cardiotoxicity screening with the xcelligence system: Effects of reference compounds in human iPSC-versus mouse eSC-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 2013, 68, E9–E10. [Google Scholar] [CrossRef]
- Xi, B.A.; Wang, T.X.; Li, N.; Ouyang, W.; Zhang, W.; Wu, J.Y.; Xu, X.; Wang, X.B.; Abassi, Y.A. Functional cardiotoxicity profiling and screening using the xCELLigence RTCA cardio system. J. Assoc. Lab. Autom. 2011, 16, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, C.; Unsworth, C.P.; Graham, E.S. Enrichment of differentiated hNT neurons and subsequent analysis using flow-cytometry and xCELLigence sensing. J. Neurosci. Methods 2014, 227, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Bruening-Wright, A.; Obejero-Paz, C.; Kojukhova, M.; Brown, A. Quantification of cardioactive drug effects using xCELLigence RTCA cardio and human stem cell-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 2013, 68, E6. [Google Scholar] [CrossRef]
- Golke, A.; Cymerys, J.; Slonska, A.; Dzieciatkowski, T.; Chmielewska, A.; Tucholska, A.; Banbura, M.W. The xCELLigence system for real-time and label-free analysis of neuronal and dermal cell response to Equine Herpesvirus type 1 infection. Pol. J. Vet. Sci. 2012, 15, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Dowling, C.M.; Ors, C.H.; Kiely, P.A. Using real-time impedance-based assays to monitor the effects of fibroblast-derived media on the adhesion, proliferation, migration and invasion of colon cancer cells. Biosci. Rep. 2014, 34, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Usha, M.; Malathi, P.; Al-Sohaibani, A.S.; Chandrasekaran, M. Biofilm forming multi drug resistant staphylococcus spp. Among Patients with Conjunctivitis. Pol. J. Microbiol. 2010, 59, 233–239. [Google Scholar] [PubMed]
- Rudkin, J.K.; Laabei, M.; Edwards, A.M.; Joo, H.S.; Otto, M.; Lennon, K.L.; O’Gara, J.P.; Waterfield, N.R.; Massey, R.C. Oxacillin alters the toxin expression profile of community-associated methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Ueda, O.; Tanaka, S.; Nagasawa, Z.; Hanaki, H.; Shobuike, T.; Miyamoto, H. Development of a novel matrix-assisted laser desorption/ionization time-of-flight mass spectrum (MALDI-TOF-MS)-based typing method to identify meticillin-resistant Staphylococcus aureus clones. J. Hosp. Infect. 2015, 90, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Josten, M.; Reif, M.; Szekat, C.; Al-Sabti, N.; Roemer, T.; Sparbier, K.; Kostrzewa, M.; Rohde, H.; Sahl, H.G.; Bierbaum, G. Analysis of the Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrum of Staphylococcus aureus Identifies Mutations That Allow Differentiation of the Main Clonal Lineages. J. Clin. Microbiol. 2013, 51, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Zambelli, B.; Musiani, F.; Ciurli, S. Metal Ion-Mediated DNA-Protein Interactions. In Interplay between Metal Ions and Nucleic Acids; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 10, pp. 135–170. [Google Scholar]
- Bovenkamp, G.L.; Zanzen, U.; Krishna, K.S.; Hormes, J.; Prange, A. X-Ray absorption near-edge structure (XANES) spectroscopy study of the interaction of silver ions with Staphylococcus aureus, Listeria monocytogenes, and Escherichia coli. Appl. Environ. Microbiol. 2013, 79, 6385–6390. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Rao, D.N. Role of DNA methyltransferases in epigenetic regulation in bacteria. Subcell. Biochem. 2013, 61, 81–102. [Google Scholar] [PubMed]
- Gopal, J.; Manikandan, M.; Hasan, N.; Lee, C.H.; Wu, H.F. A comparative study on the mode of interaction of different nanoparticles during MALDI-MS of bacterial cells. J. Mass Spectrom. 2013, 48, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Kang, O.H.; Lee, Y.S.; Oh, Y.C.; Chae, H.S.; Jang, H.J.; Shin, D.W.; Kwon, D.Y. Antibacterial Activity of Methyl Gallate Isolated from Galla Rhois or Carvacrol Combined with Nalidixic Acid Against Nalidixic Acid Resistant Bacteria. Molecules 2009, 14, 1773–1780. [Google Scholar] [CrossRef]
- Patel, J.B.; Eliopoulos, G.M.; Hindler, J.A.; Jenkins, S.G.; Lewis, J.S.; Limbago, B.; Miller, L.A.; Nicolau, D.P.; Powell, M.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cihalova, K.; Chudobova, D.; Michalek, P.; Moulick, A.; Guran, R.; Kopel, P.; Adam, V.; Kizek, R. Staphylococcus aureus and MRSA Growth and Biofilm Formation after Treatment with Antibiotics and SeNPs. Int. J. Mol. Sci. 2015, 16, 24656-24672. https://doi.org/10.3390/ijms161024656
Cihalova K, Chudobova D, Michalek P, Moulick A, Guran R, Kopel P, Adam V, Kizek R. Staphylococcus aureus and MRSA Growth and Biofilm Formation after Treatment with Antibiotics and SeNPs. International Journal of Molecular Sciences. 2015; 16(10):24656-24672. https://doi.org/10.3390/ijms161024656
Chicago/Turabian StyleCihalova, Kristyna, Dagmar Chudobova, Petr Michalek, Amitava Moulick, Roman Guran, Pavel Kopel, Vojtech Adam, and Rene Kizek. 2015. "Staphylococcus aureus and MRSA Growth and Biofilm Formation after Treatment with Antibiotics and SeNPs" International Journal of Molecular Sciences 16, no. 10: 24656-24672. https://doi.org/10.3390/ijms161024656
APA StyleCihalova, K., Chudobova, D., Michalek, P., Moulick, A., Guran, R., Kopel, P., Adam, V., & Kizek, R. (2015). Staphylococcus aureus and MRSA Growth and Biofilm Formation after Treatment with Antibiotics and SeNPs. International Journal of Molecular Sciences, 16(10), 24656-24672. https://doi.org/10.3390/ijms161024656