MyoD Is a Novel Activator of Porcine FIT1 Gene by Interacting with the Canonical E-Box Element during Myogenesis
Abstract
:1. Introduction
2. Results
2.1. FIT1 Expression in the Developing Skeletal Muscle and the Differentiated C2C12 Cells
2.2. Sequence Analysis of the Porcine FIT1 5′-Flanking Region


2.3. Transcriptional Activation of Porcine FIT1 Gene in Differentiated C2C12 Cells


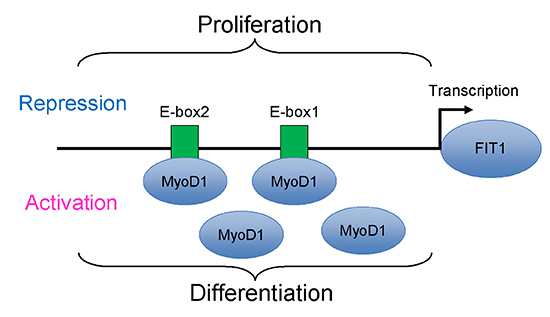
2.4. The E-Box Elements in the Core Promoter Are Required for Porcine FIT1 Transcriptional Activation during C2C12 Myogenesis
2.5. The Canonical E-Box1 Element within Porcine FIT1 Promoter Plays the Dominant Role in this Activation during C2C12 Myogenesis
2.6. MyoD1 Promotes Porcine FIT1 Transcription during C2C12 Differentiation




2.7. Binding of MyoD1 to the Canonical E-Box1 Element within Porcine FIT1 Promoter in C2C12 Myotubes
3. Discussion

4. Experimental Section
4.1. Isolation of the Porcine FIT1 5′-Flanking Region
4.2. RNA Extraction, cDNA Synthesis and qRT-PCR
4.3. Luciferase Vector Construction, Cell Culture and Transfection
4.4. Assay of the Luciferase Activity
4.5. siRNA Interference
4.6. Chromatin Immunoprecipitation (ChIP) Assay
4.7. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kadereit, B.; Kumar, P.; Wang, W.J.; Miranda, D.; Snapp, E.L.; Severina, N.; Torregroza, I.; Evans, T.; Silver, D.L. Evolutionarily conserved gene family important for fat storage. Proc. Natl. Acad. Sci. USA 2008, 105, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Li, H.J.; He, Z.F.; Wang, T.; Qin, G. Study on the flavor contribution of phospholipids and triglycerides to pork. Food Sci. Biotechnol. 2010, 19, 1267–1276. [Google Scholar] [CrossRef]
- Li, D.Z.; He, J.X.; Lei, M.G.; Xu, D.Q.; Jiang, S.W.; Xiong, Y.Z. Polymorphism in exon 2 of pig FIT1 gene and its association with fat-deposition-related traits. Yi Chuan 2010, 32, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Z. Molecular Biological Analysis of Porcine Fat-Inducing Transcript Gene. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2010. [Google Scholar]
- Emerson, C.J., Jr.; Chen, J.; King, O.D. Composition and Methods for Characterizing and Treating Muscular Dystrophy. US Patent 2013/0347136 A1, 2013. [Google Scholar]
- Gross, D.A.; Snapp, E.L.; Silver, D.L. Structural insights into triglyceride storage mediated by fat storage-inducing transmembrane (FIT) protein 2. PLoS ONE 2010, 5, e10796. [Google Scholar] [CrossRef] [PubMed]
- Gross, D.A.; Zhan, C.; Silver, D.L. Direct binding of triglyceride to fat storage-inducing transmembrane proteins 1 and 2 is important for lipid droplet formation. Proc. Natl. Acad. Sci. USA 2011, 108, 19581–19586. [Google Scholar] [CrossRef] [PubMed]
- Etzioni, S.; Yafe, A.; Khateb, S.; Weisman-Shomer, P.; Bengal, E.; Fry, M. Homodimeric MyoD preferentially binds tetraplex structures of regulatory sequences of muscle-specific genes. J. Biol. Chem. 2005, 280, 26805–26812. [Google Scholar] [CrossRef] [PubMed]
- Lassar, A.B.; Buskin, J.N.; Lockshon, D.; Davis, R.L.; Apone, S.; Hauschka, S.D.; Weintraub, H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell 1989, 58, 823–831. [Google Scholar] [CrossRef]
- Tapscott, S.J.; Weintraub, H. MyoD and the regulation of myogenesis by helix-loop-helix proteins. J. Clin. Investig. 1991, 87, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lin, L.; Bolund, L.; Jensen, T.G.; Sorensen, C.B. Genetically modified pigs for biomedical research. J. Inherit. Metab. Dis. 2012, 35, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Angione, A.R.; Jiang, C.; Pan, D.; Wang, Y.X.; Kuang, S. PPARδ regulates satellite cell proliferation and skeletal muscle regeneration. Skelet. Muscle 2011, 1, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Karlssona, A.H.; Klontb, R.E.; Fernandez, X. Skeletal muscle fibres as factors for pork quality. Livest. Prod. Sci. 1999, 60, 255–269. [Google Scholar] [CrossRef]
- Lefaucheur, L.; Vigneron, P. Post-natal changes in some histochemical and enzymatic characteristics of three pig muscles. Meat Sci. 1986, 16, 199–216. [Google Scholar] [CrossRef]
- Joo, S.T.; Kim, G.D.; Hwang, Y.H.; Ryu, Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013, 95, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Essén-Gustavsson, B.; Karlstrom, K.; Lundstrom, K.; Enfalt, A.C. Intramuscular fat and muscle fibre lipid contents in halothane-gene-free pigs fed high or low protein diets and its relation to meat quality. Meat Sci. 1994, 38, 269–277. [Google Scholar] [CrossRef]
- Picard, B.; Berri, C.; Lefaucheur, L.; Molette, C.; Sayd, T.; Terlouw, C. Skeletal muscle proteomics in livestock production. Brief. Funct. Genom. 2010, 9, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, H.; Davis, R.; Lockshon, D.; Lassar, A. MyoD binds cooperatively to two sites in a target enhancer sequence: Occupancy of two sites is required for activation. Proc. Natl. Acad. Sci. USA 1990, 87, 5623–5627. [Google Scholar] [CrossRef] [PubMed]
- Yafe, A.; Etzioni, S.; Weisman-Shomer, P.; Fry, M. Formation and properties of hairpin and tetraplex structures of guanine-rich regulatory sequences of muscle-specific genes. Nucleic Acids Res. 2005, 33, 2887–2900. [Google Scholar] [CrossRef] [PubMed]
- Shklover, J.; Etzioni, S.; Weisman-Shomer, P.; Yafe, A.; Bengal, E.; Fry, M. MyoD uses overlapping but distinct elements to bind E-box and tetraplex structures of regulatory sequences of muscle-specific genes. Nucleic Acids Res. 2007, 35, 7087–7095. [Google Scholar] [CrossRef] [PubMed]
- Shklover, J.; Weisman-Shomer, P.; Yafe, A.; Fry, M. Quadruplex structures of muscle gene promoter sequences enhance in vivo MyoD-dependent gene expression. Nucleic Acids Res. 2010, 38, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Winokur, S.T.; Chen, Y.W.; Masny, P.S.; Martin, J.H.; Ehmsen, J.T.; Tapscott, S.J.; van der Maarel, S.M.; Hayashi, Y.; Flanigan, K.M. Expression profiling of FSHD muscle supports a defect in specific stages of myogenic differentiation. Hum. Mol. Genet. 2003, 12, 2895–2907. [Google Scholar] [CrossRef] [PubMed]
- Mormeneo, E.; Jimenez-Mallebrera, C.; Palomer, X.; de Nigris, V.; Vazquez-Carrera, M.; Orozco, A.; Nascimento, A.; Colomer, J.; Lerin, C.; Gomez-Foix, A.M. PGC-1α induces mitochondrial and myokine transcriptional programs and lipid droplet and glycogen accumulation in cultured human skeletal muscle cells. PLoS ONE 2012, 7, e29985. [Google Scholar] [CrossRef] [PubMed]
- Finck, B.N.; Kelly, D.P. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J. Clin. Investig. 2006, 116, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Dodou, E.; Xu, S.M.; Black, B.L. mef2c Is activated directly by myogenic basic helix-loop-helix proteins during skeletal muscle development in vivo. Mech. Dev. 2003, 120, 1021–1032. [Google Scholar] [CrossRef]
- Molkentin, J.D.; Black, B.L.; Martin, J.F.; Olson, E.N. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell 1995, 83, 1125–1136. [Google Scholar] [CrossRef]
- Mal, A.; Harter, M.L. MyoD is functionally linked to the silencing of a muscle-specific regulatory gene prior to skeletal myogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Michael, L.F.; Wu, Z.; Cheatham, R.B.; Puigserver, P.; Adelmant, G.; Lehman, J.J.; Kelly, D.P.; Spiegelman, B.M. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc. Natl. Acad. Sci. USA 2001, 98, 3820–3825. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Noh, O.J.; Park, Y.H.; Chung, Y.W.; Kim, I.Y. Transcriptional regulation of selenoprotein W by MyoD during early skeletal muscle differentiation. J. Biol. Chem. 2010, 285, 40496–40507. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.; Xia, X.; He, J.; Ren, Z.; Xu, D.; Xiong, Y.; Zuo, B. MyoD Is a Novel Activator of Porcine FIT1 Gene by Interacting with the Canonical E-Box Element during Myogenesis. Int. J. Mol. Sci. 2015, 16, 25014-25030. https://doi.org/10.3390/ijms161025014
Yan C, Xia X, He J, Ren Z, Xu D, Xiong Y, Zuo B. MyoD Is a Novel Activator of Porcine FIT1 Gene by Interacting with the Canonical E-Box Element during Myogenesis. International Journal of Molecular Sciences. 2015; 16(10):25014-25030. https://doi.org/10.3390/ijms161025014
Chicago/Turabian StyleYan, Chi, Xiaoliang Xia, Junxian He, Zhuqing Ren, Dequan Xu, Yuanzhu Xiong, and Bo Zuo. 2015. "MyoD Is a Novel Activator of Porcine FIT1 Gene by Interacting with the Canonical E-Box Element during Myogenesis" International Journal of Molecular Sciences 16, no. 10: 25014-25030. https://doi.org/10.3390/ijms161025014
APA StyleYan, C., Xia, X., He, J., Ren, Z., Xu, D., Xiong, Y., & Zuo, B. (2015). MyoD Is a Novel Activator of Porcine FIT1 Gene by Interacting with the Canonical E-Box Element during Myogenesis. International Journal of Molecular Sciences, 16(10), 25014-25030. https://doi.org/10.3390/ijms161025014