Plants as Factories for Human Pharmaceuticals: Applications and Challenges
Abstract
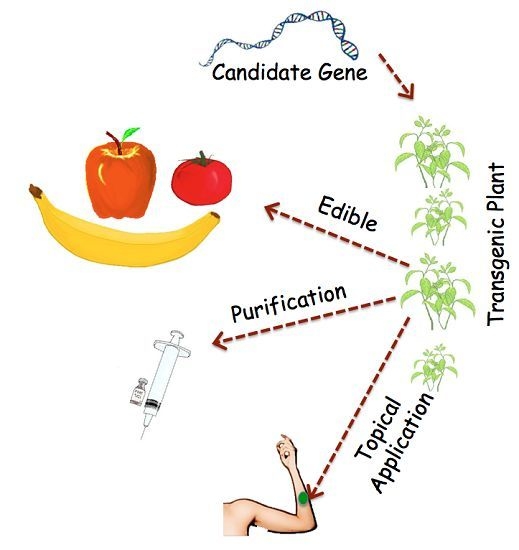
:1. Introduction
| Product | Host | Application | Clinical Trial | Status | Sponsor |
|---|---|---|---|---|---|
| Taliglucerase alfa; Recombinant glucocerebrosidase (prGCD) | Carrot cell culture | Gaucher disease | NCT00376168 | Phase 3 completed (2012); FDA approved (2012) | Protalix, Karmiel, Israel |
| ZMApp | Tobacco | Ebola Virus | NCT02363322 | Phase 1 and 2 (2015) | National Institute of Allergy and Infectious Diseases (NIAID), Bethesda, MD, USA |
| PRX-102 | Tobacco cell culture | Fabry Disease | NCT01769001 | Phase 1 and 2 (2014) | Protalix, Karmiel, Israel |
| VaccinePfs25 VLP | Tobacco | Malaria | NCT02013687 | Phase 1 (2015) | Center for Molecular Biotechnology, Plymouth, MI, USA |
| Vaccine Recombinant protective antigen | Tobacco | Anthrax | NCT02239172 | Phase 1 (2014) | Center for Molecular Biotechnology, Plymouth, MI, USA |
| HAI-05 | Tobacco | H5N1 Vaccine | NCT01250795 | Phase 1 (2011) | Center for Molecular Biotechnology, Plymouth, MI, USA |
| Recombinant human intrinsic factor | Arabidopsis thaliana | Vitamin B12 deficiency | NCT00279552 | Phase 2 Completed (2006) | University in Aarhus, Aarhus, Denmark |
| H5-VLP + GLA-AF Vaccine | Tobacco | Influenza A Subtype H5N1 Infection | NCT01657929 | Phase 1 Completed (2014) | Infectious Disease Research Institute, Seattle, WA, USA |
| P2G12 Antibody | Tobacco | HIV | NCT01403792 | Phase 1 Completed (2011) | University of Surrey, Guildford, UK |
| Company | Host | Lead Product | Expression Technology | Advantage | Website References |
|---|---|---|---|---|---|
| Mapp Biopharmaceutical/LeafBiol, USA | Tobacco leaves | ZMapp™ for Ebola crisis | MagnICON Transient expression | Speed | [2] |
| Protalix, Carmiel, Israel | Carrot or tobacco cell culture | ELELYSO™ (taliglucerase alfa) Enzyme replacement | ProCellEx® Stable Expression | Quality | [19] |
| Icon Genetics, München, Germany | Nicotiana benthamiana leaves | Vaccine for non-Hodgkin’s Lymphoma | MagnICON Transient expression | Speed and Personalization | [20] |
| Ventria Bioscience, Junction City, KS, USA | Rice seeds | VEN150 for HIV-associated chronic inflammation | Express Tec Stable Expression | Scale Cost | [21] |
| Greenovation Biotech GmbH, Heilbronn, Germany | Moss | Moss-GAA for Pompe Disease, Moss-GBA for Gaucher’s Disease, Moss-AGAL for Fabry Disease | Moss Physcomitrella patens based Broytechnolgy | Speed Scale and Customized | [22] |
| Kentucky BioProcessing, Owensboro, KY, USA | Nicotiana benthamiana leaves | Contract service | Geneware Transient expression | Speed | [23] |
| PhycoBiologics Inc. Bloomington, IN, USA | Algae | Vaccines Growth Factor and enzymes | Microalgae expression | Speed Scale | [24] |
| Medicago, Québec, QC, Canada | Nicotiana benthamiana Alfalfa | Vaccine for influenza, Pandemic market, Rabies and Rotavirus | Proficia™ Transient Expression; Stable Expression | Speed | [25] |
| Synthon, Nijmegen, The Netherlands | Duckweed LeafyBiomass | Antibody for non-Hodgkin’s Lymphoma | LEX system Stable expression | Speed Quality | [26] |
| Fraunhofer IME, Aachen, Germany | Tobacco leaves | HIV Antibody | Stable Nuclear Expression | Scale Cost | [27] |
| Fraunhofer CMB/iBio, Newark, DE, USA | Nicotiana benthamiana leaves | Influenza vaccine | Transient expression | Speed | [28] |
| Healthgen, Wuhan, Hubei, China | Rice seed | Serum albumin | Stable Expression | Quality Scale | [29] |
| PlanetBiotechnology, Hayward, CA, USA | Tobacco leaves | CaroRx for dental caries; PBI-220 antibody for anthrax; DPP4-Fc for MERS coronavirus infection | Stable Expression | Quality Scale | [4] |

2. Advantages
| Comparisons | Transgenic Plant | Plant Cell Culture | Bacteria | Yeast | Mammalian Cell Culture | Transgenic Animals |
|---|---|---|---|---|---|---|
| Overall cost | Very low | Medium | Low | Medium | High | High |
| Scale-up capacity | Very high | Medium | High | High | Very low | Low |
| Production scale | Worldwide | Limited | Limited | Limited | Limited | Limited |
| Protein yield | High | High | Medium | High | Medium-High | High |
| Protein folding accuracy | High | High | Low | Medium | High | High |
| Glycosylation | Minor differences | Minor differences | None | Incorrect | Correct | Correct |
| Product quality | High | High | Low | Medium | High | High |
| Contamination risks | Low | Low | Endotoxins | Low | Virus, Prions, oncogenic DNA | Virus, Prions, oncogenic DNA |
| Safety | High | Non-specific | Low | Unknown | Medium | High |
| Storage cost | Inexpensive | Moderate | Moderate | Moderate | Expensive | Expensive |
3. Challenges
4. PMF Products for Use as Topical Applications and Health Supplements
5. PMF Production Platforms
5.1. Transient Expression Platform

5.2. Bioreactor-Based Platforms
Plant-Cell-Culture System
5.3. Moss Culture
5.4. Algal Bioreactors
5.5. Seed-Based Platforms

6. Humanized Glycosylation in Plants for “Glycan-Better” Products
7. Downstream Processing
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Arntzen, C. Plant-made pharmaceuticals: From “Edible Vaccines” to Ebola therapeutics. Plant Biotechnol. 2015, 13, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Mapp Biopharmaceutical, Inc. Home Page. Available online: http://mappbio.com (accessed on 27 November 2015).
- World Health organization Middle East respiratory syndrome coronavirus (MERS-CoV) Updates Home Page. Available online: http://www.who.int/emergencies/mers-cov/en/ (accessed on 27 November 2015).
- Planet Biotechnology Inc. Home Page. Available online: http://www.planetbiotechnology.com (accessed on 27 November 2015).
- Barta, A.; Sommengruber, K.; Thompson, D.; Hartmuth, K.; Matzke, M.; Matzke, A. The expression of a napoline synthase human growth hormone chimeric gene in transformed tobacco and sunflower callus tissue. Plant Mol. Biol. 1986, 6, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.S.; Lam, D.M.; Arntzen, C.J. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. USA 1992, 89, 11745–11749. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Ma, J.K. Plant-made pharmaceuticals: Leading products and production platforms. Biotechnol. Appl. Biochem. 2011, 58, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Boothe, J.; Nykiforuk, C.; Shen, Y.; Zaplachinski, S.; Szarka, S.; Kuhlman, P.; Murray, E.; Morck, D.; Moloney, M.M. Seed-based expression systems for plant molecular farming. Plant Biotechnol. J. 2010, 8, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Sack, M.; Rademacher, T.; Spiegel, H.; Boes, A.; Hellwig, S.; Drossard, J.; Stoger, E.; Fischer, R. From gene to harvest: Insights into upstream process development for the GMP production of a monoclonal antibody in transgenic tobacco plants. Plant Biotechnol. J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Abiri, R.; Valdiani, A.; Maziah, M.; Shaharuddin, N.A.; Sahebi, M.; Yusof, Z.Y.; Atabaki, N.; Talei, D.A. Critical review of the concept of transgenic plants: Insights into pharmaceutical biotechnology and molecular farming. Curr. Issues Mol. Biol. 2015, 18, 21–42. [Google Scholar] [PubMed]
- Gomord, V.; Fitchette, A.C.; Menu-Bouaouiche, L.; Saint-Jore-Dupas, C.; Plasson, C.; Michaud, D.; Faye, L. Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol. J. 2010, 8, 564–587. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, K.K.; Hiss, M.; Rensing, S.A. Means to optimize protein expression in transgenic plants. Curr. Opin. Biotechnol. 2015, 32, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Kristina, K.L.; Zeng, W.; Heazlewood, J.L.; Bacic, A. Characterization of protein N-glycosylation by tandem mass spectrometry using complementary fragmentation techniques. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Buyel, J.F.; Fischer, R. A juice extractor can simplify the downstream processing of plant-derived biopharmaceutical proteins compared to blade-based homogenizers. Process Biochem. 2014, 50, 859–866. [Google Scholar] [CrossRef]
- Buyel, J.F.; Twyman, R.M.; Fischer, R. Extraction and downstream processing of plant-derived recombinant proteins. Biotechnol. Adv. 2015, 33, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Vasilev, N.; Twyman, R.M.; Schillberg, S. High-value products from plants: The challenges of process optimization. Curr. Opin. Biotechnol. 2015, 32, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.L. First plant-made biologic approved. Nat. Biotechnol. 2012, 30. [Google Scholar] [CrossRef]
- U.S. National Institutes of Health Clinical Trial Home Page. Available online: https://clinicaltrials.gov (accessed on 27 November 2015).
- Protealix Biotherapeutics Home Page. Available online: https://www.protalix.com (accessed on 27 November 2015).
- Icon Genetics GmbH Home Page. Available online: https://www.icongenetics.com (accessed on 27 November 2015).
- Ventria Biosciences Home Page. Available online: https://www.ventria.com (accessed on 27 November 2015).
- Greenovation Biotech GmbH Home Page. Available online: https://www.greenovation.com (accessed on 27 November 2015).
- Kentucky BioProcessing Home Page. Available online: https://www.kbpllc.com (accessed on 27 November 2015).
- PhycoBiologics Inc., Home Page. Available online: https://www.phycotransgenics.com (accessed on 27 November 2015).
- Medicago Home Page. Available online: https://www.medicago.com (accessed on 27 November 2015).
- Synthon Home Page. Available online: http://www.synthon.com (accessed on 27 November 2015).
- Fraunhofer IME Home Page. Available online: http://www.ime.fraunhofer.de (accessed on 27 November 2015).
- Fraunhofer CMB Home Page. Available online: http://www.fhcmb.org (accessed on 27 November 2015).
- Healthgen Biotechnology Home Page. Available online: http://www.oryzogen.com (accessed on 27 November 2015).
- Walmsley, A.M.; Arntsen, C.J. Plants for delivery of edible vaccines. Curr. Opin. Biotechnol. 2000, 22, 126–129. [Google Scholar] [CrossRef]
- Fukuda, K.; Ishida, W.; Harada, Y.; Wakasa, Y.; Takagi, H.; Takaiwa, F.; Fukushima, A. Prevention of allergic conjunctivitis in mice by a rice-based edible vaccine containing modified Japanese cedar pollen allergens. Br. J. Ophthalmol. 2015, 99, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Barzegari, A.; Saeedi, N.; Zarredar, H.; Barar, J.; Omidi, Y. The search for a promising cell factory system for production of edible vaccine. Hum. Vaccines Immunother. 2014, 10, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Na, Y.J.; Yang, B.G.; Choi, J.P.; Seo, Y.B.; Hong, C.P.; Yun, C.H.; Kim, D.H.; Sohn, E.J.; Kim, J.H.; et al. Oral immunization of haemaggulutinin H5 expressed in plant endoplasmic reticulum with adjuvant saponin protects mice against highly pathogenic avian influenza A virus infection. Plant Biotechnol. J. 2015, 13, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Mendoza, S.; Salazar-González, J.A. Immunological aspects of using plant cells as delivery vehicles for oral vaccines. Expert Rev. Vaccines 2014, 13, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Ata, A.A.; Vitti, A.; Nuzzaci, M.; El-Attar, A.K.; Piazzolla, G.; Tortorella, C.; Harandi, A.M.; Olson, O.; Wright, S.A.; Piazzolla, P. Plant-based vaccines: Novel and low-cost possible route for Mediterranean innovative vaccination strategies. Adv. Virus Res. 2014, 89, 1–37. [Google Scholar] [PubMed]
- Kong, Q.; Richter, L.; Yang, Y.F.; Arntzen, C.J.; Mason, H.S.; Thanavala, Y. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc. Natl. Acad. Sci. USA 2001, 98, 11539–11544. [Google Scholar] [CrossRef] [PubMed]
- Thanavala, Y.; Mahoney, M.; Pal, S.; Scott, A.; Richter, L.; Natarajan, N.; Goodwin, P.; Arntzen, C.J.; Mason, H.S. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc. Natl. Acad. Sci. USA 2005, 102, 3378–3382. [Google Scholar] [CrossRef] [PubMed]
- Hefferon, K. Clinical trials fuel the promise of plant-derived vaccines. Am. J. Clin. Med. 2010, 7, 30–37. [Google Scholar]
- Antibodies-online Inc. Home Page. Available online: http://www.antibodies-online.com (accessed on 27 November 2015).
- PromoKine Home Page. Available online: https://www.promokine.info (accessed on 27 November 2015).
- Spök, A.; Karner, S. Plant Molecular Farming, Opportunities and Challenges; JRC Technical Report EUR 23383 EN; Office for Official Publications of the European Communities: Kopstal, Luxembourg, 2008. [Google Scholar]
- Sabalza, M.; Christou, P.; Capell, T. Recombinant plant-derived pharmaceutical proteins: Current technical and economic bottlenecks. Biotechnol. Lett. 2014, 36, 2367–2379. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, J.; Bouché, F.; Desiron, C.; Stautemas, J.; de LemosEsteves, F.; Périlleux, C.; Tocquin, P. Extracellular peptidase hunting for improvement of protein production in plant cells and roots. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breyer, D.; Goossens, M.; Herman, P.; Sneyers, M. Biosafety considerations associated with molecular farming in genetically modified plants. J. Med. Plant Res. 2009, 3, 825–838. [Google Scholar]
- Sparrow, P.; Broer, I.; Hood, E.E.; Eversole, K.; Hartung, F.; Schiemann, J. Risk assessment and regulation of molecular farming—A comparison between Europe and US. Curr. Pharm. Des. 2013, 19, 5513–5530. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.; Doshi, K.; Dussault, M.; Hall, J.C.; Holbrook, L.; Jones, G.; Kaldis, A.; Klima, C.L.; Macdonald, P.; McAllister, T.; et al. Bringing plant-based veterinary vaccines to market: Managing regulatory and commercial hurdles. Biotechnol. Adv. 2015. [Google Scholar] [CrossRef] [PubMed]
- FDA guidance for industry: Drugs, biologics, and medical devices derived from bioengineered plants for use in humans and animals (draft guidance). FDA website. Available online: Http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/ucm055424.pdf (accessed on 27 November 2015).
- Strauss, D.M. Liability for genetically modified food: Are GMOs a tort waiting to happen? SciTech Lawyer 2012, 9, 8–13. [Google Scholar]
- Jin, C.; Altmann, F.; Strasser, R.; Mach, L.; Schähs, M.; Kunert, R.; Rademacher, T.; Glössl, J.; Steinkellner, H. A plant-derived human monoclonal antibody induces an anti-carbohydrate immune response in rabbits. Glycobiology 2008, 18, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Mari, A. IgE to cross-reactive carbohydrate determinants: Analysis of the distribution and appraisal of the in vivo and in vitro reactivity. Int. Arch. Allergy Immunol. 2002, 129, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.R.; Rodrigues, M.E.; Henriques, M.; Oliveira, R.; Azeredo, J. Glycosylation: Impact, control and improvement during therapeutic protein production. Crit. Rev. Biotechnol. 2014, 34, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Bardor, M.; Faveeuw, C.; Fitchette, A.C.; Gilbert, D.; Galas, L.; Trottein, F.; Faye, L.; Lerouge, P. Immunoreactivity in mammals of two typical plant glyco-epitopes, core alpha(1,3)-fucose and core xylose. Glycobiology 2003, 13, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Castilho, A.; Steinkellner, H. Glyco-engineering in plants to produce human-like N-glycan structures. Biotechnol. J. 2012, 7, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.K.C.; Hikmat, B.Y.; Wycoff, K.; Vine, N.D.; Chargelegue, D.; Yu, L.; Hein, M.B.; Lherner, T. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat. Med. 1998, 4, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, L.; Olmsted, S.S.; Moench, T.C.; Co, M.S.; Martinell, B.J.; Paradkar, V.M.; Russell, D.R.; Queen, C.; Cone, R.A.; Whaley, K. A humanized monoclonal antibody produced in transgenic plants for immunoprotection of the vagina against genital herpes. Nat. Biotechnol. 1998, 16, 1361–1364. [Google Scholar] [PubMed]
- Tan, Y.; Wang, K.Y.; Wang, N.; Li, G.; Liu, D. Ectopic expression of human acidic fibroblast growth factor 1 in the medicinal plant, Salvia miltiorrhiza, accelerates the healing of burn wounds. BMC Biotechnol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Ji, J.; Jin, C.; Wang, G.; Li, X.; Guan, W. Expression of cholera toxin B subunit-lumbrokinase in edible sunflower seeds-the use of transmucosal carrier to enhance its fusion protein’s effect on protection of rats and mice against thrombosis. Biotechnol. Prog. 2014, 30, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Du, X.; Wang, G.; Ji, J.; Jin, C.; Li, X. Expression of biologically active anti-thrombosis protein lumbrokinase in edible sunflower seed kernel. J. Plant. Biochem. Biotechnol. 2014, 23, 257–265. [Google Scholar] [CrossRef]
- Wang, K.; Tull, L.; Cooper, E.; Wang, N.; Liu, D. Recombinant protein production of earthworm lumbrokinase for potential antithrombotic application. Evid. Based Complement. Altern. Med. 2013, 783971. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.B.; Curtis, W.R. Comparison of transient protein expression in tobacco leaves and plant suspension culture. Biotechnol. Prog. 2005, 21, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, F.; Lomonossoff, G.P. Transient expressions of synthetic biology in plants. Curr. Opin. Plant Biol. 2014, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Peyret, H.; Lomonossoff, G.P. When plant virology met Agrobacterium: The rise of the deconstructed clones. Plant Biotechnol. J. 2015, 13, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Catrice, E.V.; Sainsbury, F. Assembly and purification of polyomavirus-like particles from plants. Mol. Biotechnol. 2015, 57, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Phoolcharoen, W.; Lai, H.; Piensook, K.; Cardineau, G.; Zeitlin, L.; Whaley, K.J.; Arntzen, C.J.; Mason, H.S.; Chen, Q. High-level rapid production of full-size monoclonal antibodies in plants by a single-vector DNA replicon system. Biotechnol. Bioeng. 2010, 106, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Wong, G.; Audet, J.; Bello, A.; Fernando, L.; Alimonti, J.B.; Fausther-Bovendo, H.; Wei, H.; Aviles, J.; Hiatt, E.; et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 2014, 514, 47–53. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health & Human Service: Partnership advances experimental Ebola drug. Available online: http://www.hhs.gov/about/news/2015/07/20/hhs-partnership-advances-experimental-ebola-drug.html (accessed on 27 November 2015).
- Schillberg, S.; Raven, N.; Fischer, R.; Twyman, R.M.; Schiermeyer, A. Molecular farming of pharmaceutical proteins using plant suspension cell and tissue cultures. Curr. Pharm. 2013, 19, 5531–5542. [Google Scholar] [CrossRef]
- Magy, B.; Tollet, J.; Laterre, R.; Boutry, M.; Navarre, C. Accumulation of secreted antibodies in plant cell cultures varies according to the isotype, host species and culture conditions. Plant Biotechnol. J. 2014, 12, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Raven, N.; Rasche, S.; Kuehn, C.; Anderlei, T.; Klöckner, W.; Schuster, F.; Henquet, M.; Bosch, D.; Büchs, J.; Fischer, R.; et al. Scaled-up manufacturing of recombinant antibodies produced by plant cells in a 200-L orbitally-shaken disposable bioreactor. Biotechnol. Bioeng. 2015, 112, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Tekoah, Y.; Shulman, A.; Kizhner, T.; Ruderfer, I.; Fux, L.; Nataf, Y.; Bartfeld, D.; Ariel, T.; Gingis-Velitski, S.; Hanania, U.; et al. Large-scale production of pharmaceutical proteins in plant cell culture-the protalix experience. Plant Biotechnol. J. 2015, 13, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.K.; McDonald, K.A. Bioreactor systems for in vitro production of foreign proteins using plant cell cultures. Biotechnol. Adv. 2012, 30, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Kircheis, R.; Halanek, N.; Koller, I.; Jost, W.; Schuster, M.; Gorr, G.; Hajszan, K.; Nechansky, A. Correlation of ADCC activity with cytokine release induced by the stably expressed, glyco-engineered humanized Lewis Y-specific monoclonal antibody MB314. MABS 2012, 4, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Reski, R.; Parsons, J.; Decker, E.L. Moss-made pharmaceuticals: From bench to bedside. Plant Biotechnol. J. 2015, 13, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Niederkrüger, H.; Dabrowska-Schlepp, P.; Schaaf, A. Suspension culture of plant cells under phototrophic conditions. In Industrial Scale Suspension Culture of Living Cells; Meyer, H.P., Schmidhalter, D.R., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 259–292. [Google Scholar]
- Anterola, A.; Shanle, E.; Perroud, P.F.; Quatrano, R. Production of taxa-4(5), 11(12)-diene by transgenic Physcomitrella patens. Transgenic Res. 2009, 18, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Escobedo, L.; Rosales-Mendoza, S.; Romero-Maldonado, A.; Parsons, J.; Decker, E.L.; Monreal-Escalante, E.; Moreno-Fierros, L.; Reski, R. An Env-derived multi-epitope HIV chimeric protein produced in the moss Physcomitrella patens is immunogenic in mice. Plant Cell Rep. 2015, 34, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Infante, N.; Govea-Alonso, D.O.; Alpuche-Solís, Á.G.; García-Hernández, A.L.; Soria-Guerra, R.E.; Paz-Maldonado, L.M.; Ilhuicatzi-Alvarado, D.; Varona-Santos, J.T.; Verdín-Terán, L.; Korban, S.S.; et al. Chloroplast-derived C4V3 polypeptide from the human immunodeficiency virus (HIV) is orally immunogenic in mice. Plant Mol. Biol. 2012, 78, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.M.; Sterling, J.D.; Regan, J.T.; Gasdaska, J.R.; Frantz, K.K.; Peele, C.G.; Black, A.; Passmore, D.; Moldovan-Loomis, C.; Srinivasan, M.; et al. Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat. Biotechnol. 2006, 24, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Lin, H.; Jiang, P. Advances in genetic engineering of marine algae. Biotechnol. Adv. 2012, 30, 1602–1613. [Google Scholar] [CrossRef] [PubMed]
- Mathieu-Rivet, E.; Kiefer-Meyer, M.-C.; Vanier, G.; Ovide, C.; Burel, C.; Lerouge, P.; Bardor, M. Protein N-glycosylation in eukaryotic microalgae and its impact on the production of nuclear expressed biopharmaceuticals. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Oyler, G.A. Green algae as a platform to express therapeutic proteins. Discov. Med. 2009, 8, 28–30. [Google Scholar] [PubMed]
- Gong, Y.; Hu, H.; Gao, Y.; Xu, X.; Gao, H. Microalgae as platforms for production of recombinant proteins and valuable compounds: Progress and prospects. J. Ind. Microbiol. Biotechnol. 2011, 38, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Rasala, B.A.; Mayfield, S.P. The microalga Chlamydomonasreinhardtii as a platform for the production of human protein therapeutics. Bioeng. Bugs 2011, 2, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Qin, S.; Liu, J.; Yu, D.; Liang, C.; Tseng, C. Transient expression of lacZ in bombarded unicellular green alga Haematococcuspluvialis. J. Appl. Phycol. 2002, 14, 497–500. [Google Scholar]
- Jarvis, E.E.; Brown, L.M. Transient expression of firefly luciferase in protoplasts of the green alga Chlorella ellipsoidea. Curr. Genet. 1991, 19, 317–321. [Google Scholar] [CrossRef]
- Hawkins, R.L.; Nakamura, M. Expression of human growth hormone by the eukaryotic alga, Chlorella. Curr. Microbiol. 1999, 38, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Eichler-Stahlberg, A.; Weisheit, W.; Ruecker, O.; Heitzer, M. Strategies to facilitate transgene expression in Chlamydomonas reinhardtii. Planta 2009, 229, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Phoolcharoen, W.; Bhoo, S.; Lai, H.; Ma, J.; Arntzen, C.J.; Chen, Q.; Mason, H.S. Expression of an immunogenic Ebola immune complex in Nicotianabenthamiana. Plant Biotechnol. J. 2011, 9, 807–816. [Google Scholar] [CrossRef] [PubMed]
- De Jaeger, G.; Scheffer, S.; Jacobs, A.; Zambre, M.; Zobell, O.; Goossens, A.; Depicker, A.; Angenon, G. Boosting heterologous protein production in transgenic dicotyledonous seeds using Phaseolus vulgaris regulatory sequences. Nat. Biotechnol. 2002, 20, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Lee, K.J.; Shao, Y.; Lee, J.H.; So, Y.; Choo, Y.K.; Oh, D.B.; Hwang, K.A.; Oh, S.H.; Han, Y.S.; Ko, K. Expression of GA733-Fc fusion protein as a vaccine candidate for colorectal cancer in transgenic plants. J. Biomed. Biotechnol. 2012, 364240. [Google Scholar] [CrossRef] [PubMed]
- Downing, W.L.; Galpin, J.D.; Clemens, S.; Lauzon, S.M.; Samuels, A.L.; Pidkowich, M.S.; Clarke, A.; Kermode, A.R. Synthesis of enzymatically active human α-l-iduronidase in Arabidopsiscgl (complex glycan-deficient) seeds. Plant Biotechnol. J. 2006, 4, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Guo, Z.; Shi, J.; Wang, X.; Liu, J.; Shi, B.; Guo, F.; Zhang, C.; Yang, D. Transgenic rice endosperm as a bioreactor for molecular pharming. Plant Cell Rep. 2014, 33, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Gu, Z.; Glatz, C.E. Extraction of recombinant dog gastric lipase from transgenic corn seed. J. Agric. Food Chem. 2006, 54, 8086–8092. [Google Scholar] [CrossRef] [PubMed]
- Huang, N. High-level protein expression system uses self-pollinating crops as hosts. BioProcess Int. 2004, 2, 54–59. [Google Scholar]
- Scheller, J.; Leps, M.; Conrad, U. Forcing single-chain variable fragment production in tobacco seeds by fusion to elastin-like polypeptides. Plant Biotechnol. J. 2006, 4, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Petruccelli, S.; Otegui, M.S.; Lareu, F.; Tran Dinh, O.; Fitchette, A.C.; Circosta, A.; Rumbo, M.; Bardor, M.; Carcamo, R.; Gomord, V.; et al. A KDEL-tagged monoclonal antibody is efficiently retained in the endoplasmic reticulum in leaves, but is both partially secreted and sorted to protein storage vacuoles in seeds. Plant Biotechnol. J. 2006, 4, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Twyman, R.M.; Arcalis, E.; Stoger, E. Using storage organelles for the accumulation and encapsulation of recombinant proteins. Biotechnol. J. 2012, 7, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Loos, A.; Steinkellner, H. Plant glyco-biotechnology on the way to synthetic biology. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Stoger, E.; Fischer, R.; Moloney, M.; Ma, J.K. Plant molecular pharming for the treatment of chronic and infectious diseases. Annu. Rev. Plant Biol. 2014, 65, 743–768. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, K.; Makhzoum, A.; Trémouillaux-Guiller, J. Molecular farming on rescue of pharma industry for next generations. Crit. Rev. Biotechnol. 2015, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Ooievaar-de-Heer, P.; Scala, E.; Giani, M.; Pirrotta, L.; Zuidmeer, L.; Bethell, D.; van Ree, R. Evaluation by double-blind placebo-controlled oral challenge of the clinical relevance of IgE antibodies against plant glycans. Allergy 2008, 63, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Floss, D.M.; Sack, M.; Arcalis, E.; Stadlmann, J.; Quendler, H.; Rademacher, T.; Stoger, E.; Scheller, J.; Fischer, R.; Conrad, U. Influence of elastin-like peptide fusions on the quantity and quality of a tobacco-derived human immunodeficiency virus-neutralizing antibody. Plant Biotechnol. J. 2009, 7, 899–913. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.; Altmann, F.; Mach, L.; Glössl, J.; Steinkellner, H. Generation of Arabidopsis thaliana plants with complex N-glycans lacking β1,2-linked xylose and core α1,3-linked fucose. FEBS Lett. 2004, 561, 132–136. [Google Scholar] [CrossRef]
- Sourrouille, C.; Marquet-Blouin, E.; D’Aoust, M.A.; Kiefer-Meyer, M.C.; Séveno, M.; Pagny-Salehabadi, S.; Bardor, M.; Durambur, G.; Lerouge, P.; Vezina, L.; et al. Down-regulated expression of plant-specific glycoepitopes in alfalfa. Plant Biotechnol. J. 2008, 6, 702–721. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.; Stadlmann, J.; Schähs, M.; Stiegler, G.; Quendler, H.; Mach, L.; Glössl, J.; Weterings, K.; Pabst, M.; Steinkellner, H. Generation of glyco-engineered Nicotianabenthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol. J. 2008, 6, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Wilken, L.R.; Nikolov, Z.L. Recovery and purification of plant-made recombinant proteins. Biotechnol. Adv. 2012, 30, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q. Expression and purification of pharmaceutical proteins in plants. Biol. Eng. 2008, 1, 291–321. [Google Scholar] [CrossRef]
- Caliber Biotherapeutics Homepage. Available online: http://www.caliberbio.com (accessed on 27 November 2015).
- Holtz, B.R.; Berquist, B.R.; Bennett, L.D.; Kommineni, V.J.M.; Munigunti, R.K.; White, E.L.; Wilkerson, D.C.; Wong, K.I.; Ly, L.H.; Marcel, S. Commercial-scale biotherapeutics manufacturing facility for plant-made pharmaceuticals. Plant Biotechnol. 2015, 13, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Weng, Y.; Dickey, A.; Wang, K.Y. Plants as Factories for Human Pharmaceuticals: Applications and Challenges. Int. J. Mol. Sci. 2015, 16, 28549-28565. https://doi.org/10.3390/ijms161226122
Yao J, Weng Y, Dickey A, Wang KY. Plants as Factories for Human Pharmaceuticals: Applications and Challenges. International Journal of Molecular Sciences. 2015; 16(12):28549-28565. https://doi.org/10.3390/ijms161226122
Chicago/Turabian StyleYao, Jian, Yunqi Weng, Alexia Dickey, and Kevin Yueju Wang. 2015. "Plants as Factories for Human Pharmaceuticals: Applications and Challenges" International Journal of Molecular Sciences 16, no. 12: 28549-28565. https://doi.org/10.3390/ijms161226122
APA StyleYao, J., Weng, Y., Dickey, A., & Wang, K. Y. (2015). Plants as Factories for Human Pharmaceuticals: Applications and Challenges. International Journal of Molecular Sciences, 16(12), 28549-28565. https://doi.org/10.3390/ijms161226122