Intravenous Administration of Cilostazol Nanoparticles Ameliorates Acute Ischemic Stroke in a Cerebral Ischemia/Reperfusion-Induced Injury Model
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Particles Size and Stability of Dispersions Containing CLZ Nanoparticles
| Formulation | Content (w/v %) | Treatment | ||
|---|---|---|---|---|
| CLZ Microparticles | MC | DS | ||
| CLZmicro | 0.5 | 0.5 | 0.2 | — |
| Milled-CLZMC | 0.5 | 0.5 | — | Ball mill + Bead mill |
| Milled-CLZDS | 0.5 | — | 0.2 | Ball mill + Bead mill |
| CLZnano | 0.5 | 0.5 | 0.2 | Ball mill + Bead mill |

| Formulation | Particle Size (μm) | |
|---|---|---|
| Immediately | 14 Days after Preparation | |
| CLZmicro | 24.7 ± 14.3 | 25.1 ± 14.9 |
| Milled-CLZMC | 0.083 ± 0.062 | 0.52 ± 0.23 |
| CLZnano | 0.081 ± 0.059 | 0.086 ± 0.065 |

2.2. Evaluation of the Safety of Intravenous Injections of Dispersions Containing CLZ Nanoparticles
| A (μg/mL) | A (/min) | B (μg/mL) | β (×10−2, /min) | AUCCLZ (μg·min/mL) | MRTCLZ (min) | |
|---|---|---|---|---|---|---|
| CLZ solution | 3.87 ± 0.27 | 0.16 ± 0.01 | 1.27 ± 0.12 | 3.41 ± 0.52 | 60.0 ± 6.9 | 21.6 ± 1.7 |
| CLZnano dispersion | 3.85 ± 0.41 | 0.17 ± 0.01 | 1.31 ± 0.10 | 3.08 ± 0.24 | 57.1 ± 8.1 | 24.1 ± 2.7 |



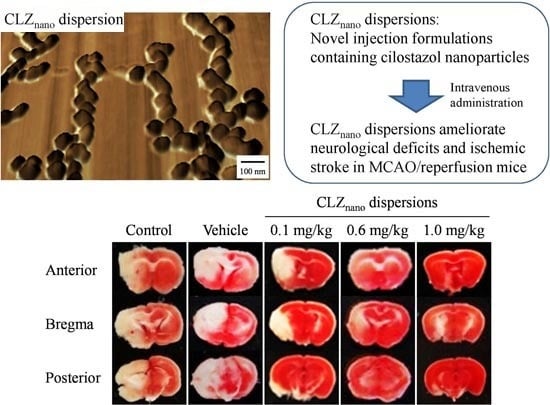
2.3. Protective Effect of the Intravenous Injection of Dispersions Containing CLZ Nanoparticles on Ischemic Stroke in MCAO/Reperfusion Mice


3. Discussion
4. Materials and Methods
4.1. Animals and Materials
4.2. Preparation of Dispersions Containing CLZ Nanoparticles
4.3. Stability of Dispersions Containing CLZ
4.4. Hemolysis of Rabbit Red Blood Cells (RBC) by Treatment with Dispersions Containing CLZ Nanoparticles
4.5. Induction of Focal Cerebral Ischemia/Reperfusion
4.6. Neurological Deficits
4.7. Assay of Plasma CLZ Concentrations
4.8. CLZ Concentration in Brain
4.9. Measurement of Blood Pressure
4.10. Measurement of Blood Flow in Carotid Artery and Brain
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Go, A.S. The epidemiology of atrial fibrillation in elderly persons: The tip of the iceberg. Am. J. Geriatr. Cardiol. 2005, 14, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Nito, C.; Kamada, H.; Endo, H.; Niizuma, K.; Myer, D.J.; Chan, P.H. Role of the p38 mitogen-activated protein kinase/cytosolic phospholipase A2 signaling pathway in blood-brain barrier disruption after focal cerebral ischemia and reperfusion. J. Cereb. Blood Flow Metab. 2008, 28, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ruiz, A.; Vacio-Adame, P.; Monroy-Noyola, A.; Méndez-Armenta, M.; Ortiz-Plata, A.; Montes, S.; Rios, C. Metallothionein-II inhibits lipid peroxidation and improves functional recovery after transient brain ischemia and reperfusion in rats. Oxid. Med. Cell. Longev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Dávalos, A.; Noya, M. Progression of ischaemic stroke and excitotoxic aminoacids. Lancet 1997, 349, 79–83. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Riley, D.P.; Caputi, A.P.; Salvemini, D. Antioxidant therapy: A new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 2001, 53, 135–159. [Google Scholar] [PubMed]
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar]
- Ishiguro, M.; Mishiro, K.; Fujiwara, Y.; Chen, H.; Izuta, H.; Tsuruma, K.; Shimazawa, M.; Yoshimura, S.; Satoh, M.; Iwama, T.; et al. Phosphodiesterase-III inhibitor prevents hemorrhagic transformation induced by focal cerebral ischemia in mice treated with tPA. PLoS ONE 2010, 5, 15178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shakur, Y.; Yoshitake, M.; Kambayashi, J.-I. Cilostazol (pletal®): A dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc. Drug Rev. 2001, 19, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Shin, H.K.; Choi, J.M.; Hong, K.W. Inhibition of lipopolysaccharide induced apoptosis by cilostazol in human umbilical vein endothelial cells. J. Pharmacol. Exp. Ther. 2002, 300, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Lin, J.X.; Takahashi, R.; Tomimoto, H. Cilostazol alleviates cerebral small-vessel pathology and white-matter lesions in stroke-prone spontaneously hypertensive rats. Brain Res. 2008, 1203, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Gotoh, F.; Fukuuchi, Y.; Amano, T.; Uematsu, D.; Kawamura, J.; Yamawaki, T.; Itoh, N.; Obara, K.; Muramatsu, K. Effects of a selective inhibitor of cyclic AMP phosphodiesterase on the pial microcirculation in feline cerebral ischemia. Stroke 1989, 20, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Oyama, N.; Yagita, Y.; Kawamura, M.; Sugiyama, Y.; Terasaki, Y.; Omura-Matsuoka, E.; Sasaki, T.; Kitagawa, K. Cilostazol, not aspirin, reduces ischemic brain injury via endothelial protection in spontaneously hypertensive rats. Stroke 2011, 42, 2571–2577. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, Y.; Nakagomi, T.; Matsuyama, T.; Stern, D.; Taguchi, A. Cilostazol reduces the risk of hemorrhagic infarction after administration of tissue-type plasminogen activator in a murine stroke model. Stroke 2012, 43, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, Y.; Tsuruma, K.; Shimazawa, M.; Yoshimura, S.; Iwama, T.; Hara, H. Cilostazol protects against hemorrhagic transformation in mice transient focal cerebral ischemia-induced brain damage. Neurosci. Lett. 2009, 452, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kiwada, H. Accelerated blood clearance of PEGylated liposomes after repeated injection. Drug Deliv. Syst. 2004, 19, 495–510. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Igarashi, R.; Takenaga, M.; Takeuchi, J.; Kitagawa, A.; Matsumoto, K.; Mizushima, Y. Marked hypotensive and blood flow-increasing effects of a newlipo-PGE1 (lipo-AS013) due to vascular wall targeting. J. Control. Release 2001, 71, 157–164. [Google Scholar] [CrossRef]
- Murakami, H.; Kobayashi, M.; Takeuchi, H.; Kawashima, Y. Further application of a modified spontaneous emulsification solvent diffusion method to various types of PLGA and PLA polymers for preparation of nanoparticles. Powder Technol. 2000, 107, 137–143. [Google Scholar] [CrossRef]
- Kawashima, Y. Design of poly(lactic-co-glycolic acid) (PLGA) nanosphere for developing to DDS. J. Pharm. Sci. Technol. Jpn. 2006, 66, 224–238. [Google Scholar]
- Tsukada, Y.; Hara, K.; Bando, Y.; Huang, C.C.; Kousaka, Y.; Kawashima, Y.; Morishita, R.; Tsujimoto, H. Particle size control of poly(d,l-lactide-co-glycolide) nanospheres for sterile applications. Int. J. Pharm. 2009, 370, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Mano, Y.; Tanabe, W.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation of disulfiram prolongs corneal residence time of the drug and reduces intraocular pressure. Exp. Eye Res. 2015, 132, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, S.T. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx 2005, 2, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.R.; Kraus, K.; Batter, D.K.; Blunt, D.G.; Weremowitz, J.; Lynch, S.E.; Antoniades, H.N.; Hansson, H.A. Gel matrix vehicles for growth factor application in nerve gap injuries repaired with tubes: A comparison of biomatrix, collagen, and methylcellulose. Exp. Neurol. 1997, 146, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Tator, C.H.; Shoichet, M.S. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials 2006, 27, 2370–2379. [Google Scholar] [CrossRef] [PubMed]
- Chouly, C.; Pouliquen, D.; Lucet, I.; Jeune, J.J.; Jallet, P. Development of superparamagnetic nanoparticles for MRI: Effect of particle size, charge and surface nature on biodistribution. J. Microencapsul. 1996, 13, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Begley, D.J. Delivery of therapeutic agents to the central nervous system: The problems and the possibilities. Pharmacol. Ther. 2004, 104, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Tani, T.; Kanbe, T.; Watanabe, K. Effect of cilostazol on platelet aggregation and experimental thrombosis. Arzneim. Forsch. 1985, 35, 1144–1149. [Google Scholar]
- Minami, N.; Suzuki, Y.; Yamamoto, M.; Kihira, H.; Imai, E.; Wada, H.; Kimura, Y.; Ikeda, Y.; Shiku, H.; Nishikawa, M. Inhibition of shear stress-induced platelet aggregation by cilostazol, a specific inhibitor of cGMP-inhibited phosphodiesterase, in vitro and ex vivo. Life Sci. 1997, 61, 383–389. [Google Scholar] [CrossRef]
- Kohda, N.; Tani, T.; Nakayama, S.; Adachi, T.; Marukawa, K.; Ito, R.; Ishida, K.; Matsumoto, Y.; Kimura, Y. Effect of cilostazol, a phosphodiesterase III inhibitor, on experimental thrombosis in the porcine carotid artery. Thromb. Res. 1999, 96, 261–268. [Google Scholar] [CrossRef]
- Kawamura, K.; Watanabe, K.; Kimura, Y. Effect of cilostazol, a new antithrombotic drug, on cerebral circulation. Arzneim. Forsch. 1985, 35, 1149–1154. [Google Scholar]
- Lee, J.H.; Park, S.Y.; Lee, W.S.; Hong, K.W. Lack of antiapoptotic effects of antiplatelet drug, aspirin and clopidogrel, and antioxidant, MCI-186, against focal ischemic brain damage in rats. Neurol. Res. 2005, 27, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, K.Y.; Lee, Y.K.; Park, S.Y.; Kim, C.D.; Lee, W.S.; Rhim, B.Y.; Hong, K.W. Cilostazol prevents focal cerebral ischemic injury by enhancing casein kinase 2 phosphorylation and suppression of phosphatase and tensin homolog deleted from chromosome 10 phosphorylation in rats. J. Pharmacol. Exp. Ther. 2004, 308, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, S.Y.; Shin, H.K.; Kim, C.D.; Lee, W.S.; Hong, K.W. Protective effects of cilostazol against transient focal cerebral ischemia and chronic cerebral hypoperfusion injury. CNS Neurosci. Ther. 2008, 14, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Wakida, K.; Morimoto, N.; Shimazawa, M.; Hozumi, I.; Nagase, H.; Inuzuka, T.; Hara, H. Cilostazol reduces ischemic brain damage partly by inducing metallothionein-1 and -2. Brain Res. 2006, 1116, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Ito, Y.; Nagai, N.; Murao, T.; Takiguchi, Y.; Kurimoto, T.; Mimura, O. Preparation of ophthalmic formulations containing cilostazol as an anti-glaucoma agent and improvement in its permeability through the rabbit cornea. J. Oleo Sci. 2010, 59, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Huang, P.L.; Panahian, N.; Fishman, M.C.; Moskowitz, M.A. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J. Cereb. Blood Flow Metab. 1996, 16, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Funakami, Y.; Hata, T.; Itoh, E.; Itano, S. Effects of some β-adrenoceptor antagonists on orthostatic hypotension in repeatedly cold- (SART-) stressed rats. Biol. Pharm. Bull. 2007, 30, 303–308. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Yoshioka, C.; Ito, Y.; Funakami, Y.; Nishikawa, H.; Kawabata, A. Intravenous Administration of Cilostazol Nanoparticles Ameliorates Acute Ischemic Stroke in a Cerebral Ischemia/Reperfusion-Induced Injury Model. Int. J. Mol. Sci. 2015, 16, 29329-29344. https://doi.org/10.3390/ijms161226166
Nagai N, Yoshioka C, Ito Y, Funakami Y, Nishikawa H, Kawabata A. Intravenous Administration of Cilostazol Nanoparticles Ameliorates Acute Ischemic Stroke in a Cerebral Ischemia/Reperfusion-Induced Injury Model. International Journal of Molecular Sciences. 2015; 16(12):29329-29344. https://doi.org/10.3390/ijms161226166
Chicago/Turabian StyleNagai, Noriaki, Chiaki Yoshioka, Yoshimasa Ito, Yoshinori Funakami, Hiroyuki Nishikawa, and Atsufumi Kawabata. 2015. "Intravenous Administration of Cilostazol Nanoparticles Ameliorates Acute Ischemic Stroke in a Cerebral Ischemia/Reperfusion-Induced Injury Model" International Journal of Molecular Sciences 16, no. 12: 29329-29344. https://doi.org/10.3390/ijms161226166
APA StyleNagai, N., Yoshioka, C., Ito, Y., Funakami, Y., Nishikawa, H., & Kawabata, A. (2015). Intravenous Administration of Cilostazol Nanoparticles Ameliorates Acute Ischemic Stroke in a Cerebral Ischemia/Reperfusion-Induced Injury Model. International Journal of Molecular Sciences, 16(12), 29329-29344. https://doi.org/10.3390/ijms161226166