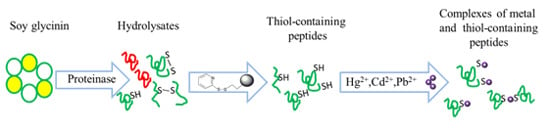
Heavy Metal Complexation of Thiol-Containing Peptides from Soy Glycinin Hydrolysates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Soy Glycinin Hydrolysates and Thiol-Containing Peptides (TCPs)



2.2. pH Titration Curves in the Absence of Metal Ions
| Hydrolysates | Alc05 | Alc15 | Alc25 | Pap05 | Pap15 | Pap25 | Pep05 | Pep15 | Pep25 |
|---|---|---|---|---|---|---|---|---|---|
| Concentration of KOH (mM) | 22.0 | 18 | 14 | 18.2 | 18.8 | 20.0 | 22.0 | 18.0 | 17.0 |
| pK | pK1 | pK2 | pK3 | pK4 | |
|---|---|---|---|---|---|
| Experimental Values | 2.3 | 3.6 | 8.9 | 9.8 | |
| Literature Values | [35] | 2.12 | 3.53 | 8.66 | 9.62 |
| [36] | 2.09 | 3.48 | 8.67 | 9.54 | |
| [37] | 1.98 | 3.49 | 8.75 | 9.69 | |
| DH/% | Alcalase | Papain | Pepsin |
|---|---|---|---|
| 5 | pK1 = 11.6, pK2 = 9.9, pK3 = 7.1, pK4 = 4.5, pK5 = 3.5, pK6 = 2.4 | pK1 = 10.7, pK2 = 7.5, pK3 = 4.4, pK4 = 3.1 | pK1 = 11.4, pK2 = 9.7, pK3 = 7.0, pK4 = 4.6, pK5 = 3.7, pK6 = 2.6 |
| 15 | pK1 = 9.9, pK2 = 7.1, pK3 = 4.3, pK4 = 3.2 | pK1 = 10.4, pK2 = 7.4, pK3 = 3.8, pK4 = 2.7 | pK1 = 10.6, pK2 = 7.9, pK3 = 4.9, pK4 = 3.6 |
| 25 | pK1 = 11.4, pK2 = 8.0, pK3 = 3.1 | pK1 = 11.1, pK2 = 8.0, pK3 = 2.7, pK4 = 1.8 | pK1 = 10.9, pK2 = 8.0, pK3 = 4.4, pK4 = 3.0 |
2.3. pH Titration Curves in the Presence of Metal Ions

| Hydrolysates | Hg2+ | Cd2+ | Pb2+ | |||
|---|---|---|---|---|---|---|
| lgβ1 | lgβ2 | lgβ1 | lgβ2 | lgβ1 | lgβ2 | |
| Alc05 | 10.4 ± 0.5 | 15.4 ± 0.7 | 12.3 ± 0.6 | 18.7 ± 0.9 | 3.8 ± 0.2 | 7.8 ± 0.4 |
| Alc15 | 16.0 ± 0.7 | 21.1 ± 1.0 | 11.4 ± 0.5 | 17.4 ± 0.7 | 10.1 ± 0.6 | 16.5 ± 0.8 |
| Alc25 | 18.0 ± 0.3 | 30.5 ± 1.3 | 14.8 ± 0.7 | 25.3 ± 1.2 | 14.1 ± 0.7 | 25.6 ± 1.1 |
| Pap05 | 17.5 ± 0.8 | 21.8 ± 0.9 | 7.8 ± 0.4 | 12.8 ± 0.6 | 7.6 ± 0.5 | 12.4 ± 0.8 |
| Pap15 | 16.8 ± 0.8 | 21.2 ± 0.9 | 7.9 ± 0.4 | 12.3 ± 0.6 | 11.7 ± 0.5 | 17.2 ± 0.7 |
| Pap25 | 19.4 ± 0.4 | 33.4 ± 1.5 | 9.2 ± 0.4 | 15.2 ± 0.8 | 13.3 ± 0.6 | 21.1 ± 0.9 |
| Pep05 | 11.6 ± 0.6 | 16.0 ± 1.0 | 10.1 ± 0.5 | 14.6 ± 0.7 | 10.4 ± 0.5 | 15.6 ± 0.8 |
| Pep15 | 24.4 ± 1.0 | 27.2 ± 0.9 | 11.7 ± 0.5 | 15.1 ± 0.8 | 10.5 ± 0.5 | 15.2 ± 0.7 |
| Pep25 | 10.6 ± 0.3 | 33.1 ± 1.0 | 12.1 ± 0.6 | 17.1 ± 0.8 | 12.6 ± 0.6 | 18.0 ± 0.7 |
2.4. Correlations between Sulfhydryl Group Content and Stability Constants


2.5. Correlations between Mw Distributions and Stability Constants
| Heavy Metals | Fraction I | Fraction II | Fraction III | Fraction IV | Fraction V | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| lgβ1 | lgβ2 | lgβ1 | lgβ2 | lgβ1 | lgβ2 | lgβ1 | lgβ2 | lgβ1 | lgβ2 | |
| Hg2+ | −0.2 | −0.581 | −0.202 | −0.395 | −0.097 | 0.122 | 0.410 | 0.815 b | 0.279 | 0.553 |
| Cd2+ | −0.379 | −0.274 | −0.028 | −0.273 | 0.187 | −0.154 | 0.197 | 0.229 | 0.243 | 0.511 |
| Pb2+ | −0.854 b | −0.762 a | −0.142 | −0.300 | 0.159 | −0.057 | 0.702 a | 0.689 a | 0.681 a | 0.835 b |
3. Experimental Section
3.1. Materials
3.2. Preparation of Soy Glycinin
3.3. Enzymatic Hydrolysis of 11S
3.4. Sulfhydryl Group Content Measurement
3.5. Total Sulfhydryl Group Measurement
3.6. TCPs Extraction
3.7. Molecular Weight Distribution by Size Exclusion Chromatography
3.8. Potentiometric Determinations
3.9. Calculation of Stability Constants
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sabolic, I. Common mechanisms in nephropathy induced by toxic metals. Nephron. Physiol. 2006, 104, 107–114. [Google Scholar] [CrossRef]
- Stohs, S.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ward, O.P. Biodegradation and Bioremediation; Springer: Berlin, Germany, 2004; p. 237. [Google Scholar]
- Bae, W.; Chen, W.; Mulchandani, A.; Mehra, R.K. Enhanced bioaccumulation of heavy metals by bacterial cells displaying synthetic phytochelatins. Biotechnol. Bioeng. 2000, 70, 518–524. [Google Scholar] [PubMed]
- Meister, A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988, 263, 17205–17208. [Google Scholar] [PubMed]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Kaegi, J.H. R.; Schaeffer, A. Biochemistry of metallothionein. Biochemistry 1988, 27, 8509–8515. [Google Scholar]
- Mahnam, B.S.; Mobini-Dehkordi, M.; Fassihi, A.; A Mohammadi, K. Design of a novel metal binding peptide by molecular dynamics simulation to sequester Cu and Zn ions. Res. Pharm. Sci. 2013, 9, 69–82. [Google Scholar]
- Grill, E.; Winnacker, E.-L.; Zenk, M.H. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc. Natl. Acad. Sci. USA 1987, 84, 439–443. [Google Scholar] [CrossRef] [PubMed]
- DeSilva, T.M.; Veglia, G.; Porcelli, F.; Prantner, A.M.; Opella, S.J. Selectivity in heavy metal-binding to peptides and proteins. Biopolymers 2002, 64, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Kaim, W.; Schwederski, B.; Klein, A. Bioinorganic Chemistry—Inorganic Elements in the Chemistry of Life: An Introduction and Guide; John Wiley & Sons: West Sussex, UK, 2013. [Google Scholar]
- Flora, S.J.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef] [PubMed]
- Ngu-Schwemlein, M.; Merle, J.K.; Healy, P.; Schwemlein, S.; Rhodes, S. Thermodynamics of the complexation of Hg(II) by cysteinyl peptide ligands using isothermal titration calorimetry. Thermochim. Acta 2009, 496, 129–135. [Google Scholar] [CrossRef]
- YOSHIDA, A.; KAPLAN, B.E.; KIMURAt, M. Metal-binding and detoxification effect of synthetic oligopeptides containing three cysteinyl residues. Proc. Natl. Acad. Sci. USA 1979, 76, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Brooks, J.; Bronson, M.; Ngu-Schwemlein, M. Evaluation of the association of mercury(II) with some dicysteinyl tripeptides. Bioorg. Chem. 2012, 44, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Ngu-Schwemlein, M.; Gilbert, W.; Askew, K.; Schwemlein, S. Thermodynamics and fluorescence studies of the interactions of cyclooctapeptides with Hg2+, Pb2+, and Cd2+. Bioorg. Med. Chem. 2008, 16, 5778–5787. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Butteiger, D.N.; Rains, T.M.; Lawless, A.; Reeves, M.S.; Schasteen, C.; Krul, E.S. Effects of soy protein on lipoprotein lipids and fecal bile acid excretion in men and women with moderate hypercholesterolemia. J. Clin. Lipidol. 2010, 4, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Moon, J.; Ko, J.; Ahn, C.; Lee, H.; Shin, J.; Park, C.; Kang, J. Novel black soy peptides with antiobesity effects: Activation of leptin-like signaling and AMP-activated protein kinase. Int. J. Obes. 2008, 32, 1161–1170. [Google Scholar] [CrossRef]
- Beermann, C.; Euler, M.; Herzberg, J.; Stahl, B. Anti-oxidative capacity of enzymatically released peptides from soybean protein isolate. Eur. Food Res. Technol. 2009, 229, 637–644. [Google Scholar] [CrossRef]
- Seber, L.E.; Barnett, B.W.; McConnell, E.J.; Hume, S.D.; Cai, J.; Boles, K.; Davis, K.R. Scalable purification and characterization of the anticancer lunasin peptide from soybean. PLoS ONE 2012, 7, e35409. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Z.; Guo, M.M.; Hua, Y.F.; Cao, D.; Zhang, C.M. Enzymatic preparation of immunomodulating hydrolysates from soy proteins. Bioresour. Technol. 2008, 99, 8873–8879. [Google Scholar] [CrossRef] [PubMed]
- Wolf, W.J. Sulfhydryl content of glycinin: effect of reducing agents. J. Agric. Food Chem. 1993, 41, 168–176. [Google Scholar] [CrossRef]
- Wang, H.; Qian, W.-J.; Chin, M.H.; Petyuk, V.A.; Barry, R.C.; Liu, T.; Gritsenko, M.A.; Mottaz, H.M.; Moore, R.J.; Camp, D.G. Characterization of the mouse brain proteome using global proteomic analysis complemented with cysteinyl-peptide enrichment. J. Proteome Res. 2006, 5, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Qian, W.J.; Chen, W.N. U.; Jacobs, J.M.; Moore, R.J.; Anderson, D.J.; Gritsenko, M.A.; Monroe, M.E.; Thrall, B.D.; Camp, D.G. Improved proteome coverage by using high efficiency cysteinyl peptide enrichment: The human mammary epithelial cell proteome. Proteomics 2005, 5, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Paulech, J.; Solis, N.; Edwards, A.V.; Puckeridge, M.; White, M.Y.; Cordwell, S.J. Large-scale capture of peptides containing reversibly oxidized cysteines by thiol-disulfide exchange applied to the myocardial redox proteome. Anal. Chem. 2013, 85, 3774–3780. [Google Scholar] [CrossRef] [PubMed]
- Dionysius, D.; Milne, J. Antibacterial peptides of bovine lactoferrin: Purification and characterization. J. Dairy Sci. 1997, 80, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, S.; Xu, J.; Zeng, M.; Song, H.; Zhao, Y. Production of cysteine-rich antimicrobial peptide by digestion of oyster (Crassostrea gigas) with alcalase and bromelin. Food Control. 2008, 19, 231–235. [Google Scholar] [CrossRef]
- Atabey, H.; Sari, H. Potentiometric, theoretical, and thermodynamic studies on equilibrium constants of aurintricarboxylic acid and determination of stability constants of its complexes with Cu2+, Ni2+, Zn2+, Co2+, Hg2+, and Pb2+ metal ions in aqueous solution. J. Chem. Eng. Data 2011, 56, 3866–3872. [Google Scholar] [CrossRef]
- Gamov, G.; Dushina, S.; Sharnin, V. Stability constants of nickel(II)-nicotinamide complexes in aqueous-ethanol solutions. Russ. J. Phys. Chem. 2014, 88, 779–782. [Google Scholar] [CrossRef]
- Leverrier, P.; Montigny, C.; Garrigos, M.; Champeil, P. Metal binding to ligands: Cadmium complexes with glutathione revisited. Anal. Biochem. 2007, 371, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Cardiano, P.; Falcone, G.; Foti, C.; Sammartano, S. Sequestration of Hg2+ by some biologically important thiols. J. Chem. Eng. Data 2011, 56, 4741–4750. [Google Scholar] [CrossRef]
- Lin, S.; He, B.; Chan, D.S.H.; Chan, P.W.H.; Leung, C.H.; Ma, D.L. A G-quadruplex-based platform for the detection of Hg2+ ions using a luminescent iridium(iii) complex. RSC Adv. 2014, 4, 54826–54831. [Google Scholar] [CrossRef]
- He, H.Z.; Leung, K.H.; Fu, W.C.; Chan, D.S.H.; Leung, C.H.; Ma, D.L. Application of a DNA-based luminescence switch-on method for the detection of mercury(II) ions in water samples from Hong Kong. Environ. Res. Lett. 2012, 7, 044032. [Google Scholar] [CrossRef]
- Mi, W.; Wang, J.; Ying, W.; Jia, W.; Cai, Y.; Qian, X. Enrichment strategy of cysteine-containing peptides based on covalent chromatography. Se Pu 2010, 28, 108–114. [Google Scholar] [PubMed]
- Rabenstein, D.L. Nuclear magnetic resonance studies of the acid-base chemistry of amino acids and peptides. I. Microscopic methylmercury-complexed glutathione. J. Am. Chem. Soc. 1973, 95, 2797–2803. [Google Scholar]
- Pillai, L.; Boss, R.D.; Greenberg, M.S. On the role of solvent in complexation equilibria. II. The acid-base chemistry of some sulfhydryl and ammonium containing amino acids in water-acetonitrile mixed solvents. J. Solut. Chem. 1979, 8, 635–646. [Google Scholar]
- Arnold, A.P.; Canty, A.J. Methylmercury(II)sulfydryl interactions. Potentiometric determination of the formation constants for complexation of methylmercury(II) by sulfhydryl containing amino acids and related molecules, including glutathione. Can. J. Chem. 1983, 61, 1428–1434. [Google Scholar]
- Khoury, R.R.; Sutton, G.J.; Hibbert, D.B.; Ebrahimi, D. Measurement and modeling of acid dissociation constants of tri-peptides containing Glu, Gly, and His using potentiometry and generalized multiplicative analysis of variance. Dalton Trans. 2013, 42, 2940–2947. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Aiba, H.; Tanaka, H. Acid dissociation constants of some histidine containing peptides and formation constants of their metal complexes. Bull. Chem. Soc. Jpn. 1974, 47, 112–117. [Google Scholar] [CrossRef]
- Bulaj, G.; Kortemme, T.; Goldenberg, D.P. Ionization-reactivity relationships for cysteine thiols in polypeptides. Biochemistry 1998, 37, 8965–8972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Vogel, H. Determination of the side chain pKa values of the lysine residues in calmodulin. J. Biol. Chem. 1993, 268, 22420–22428. [Google Scholar] [PubMed]
- Chakraborty, P.; Yao, K.M.; Chennuri, K.; Vudamala, K.; Babu, P.V.R. Interactions of mercury with different molecular weight fractions of humic substances in aquatic systems. Environ. Earth Sci. 2014, 72, 931–939. [Google Scholar] [CrossRef]
- Oram, P.D.; Fang, X.; Fernando, Q.; Letkeman, P.; Letkeman, D. The formation constants of mercury(II)-glutathione complexes. Chem. Res. Toxicol. 1996, 9, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Riener, C.K.; Kada, G.; Gruber, H.J. Quick measurement of protein sulfhydryls with Ellman’s reagent and with 4,4'-dithiodipyridine. Anal. Bioanal. Chem. 2002, 373, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, D.; Graziani, M.; Dupre, S. Determination of disulphide groups in proteins. Nature 1966, 212, 294–295. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Kong, X.; Hua, Y.; Zhou, H.; Liu, Q. Continuous hydrolysis of modified wheat gluten in an enzymatic membrane reactor. J. Sci. Food Agric. 2011, 91, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Avinashi, B.; Dwivedi, C.; Banerji, S. Potentiometric studies on chelate formation of ammonium aurintricarboxylate with bivalent copper. J. Inorg. Nucl. Chem. 1970, 32, 2641–2644. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Hua, Y.; Chen, Y.; Zhang, C.; Kong, X. Heavy Metal Complexation of Thiol-Containing Peptides from Soy Glycinin Hydrolysates. Int. J. Mol. Sci. 2015, 16, 8040-8058. https://doi.org/10.3390/ijms16048040
Ding X, Hua Y, Chen Y, Zhang C, Kong X. Heavy Metal Complexation of Thiol-Containing Peptides from Soy Glycinin Hydrolysates. International Journal of Molecular Sciences. 2015; 16(4):8040-8058. https://doi.org/10.3390/ijms16048040
Chicago/Turabian StyleDing, Xiuzhen, Yufei Hua, Yeming Chen, Caimeng Zhang, and Xiangzhen Kong. 2015. "Heavy Metal Complexation of Thiol-Containing Peptides from Soy Glycinin Hydrolysates" International Journal of Molecular Sciences 16, no. 4: 8040-8058. https://doi.org/10.3390/ijms16048040
APA StyleDing, X., Hua, Y., Chen, Y., Zhang, C., & Kong, X. (2015). Heavy Metal Complexation of Thiol-Containing Peptides from Soy Glycinin Hydrolysates. International Journal of Molecular Sciences, 16(4), 8040-8058. https://doi.org/10.3390/ijms16048040