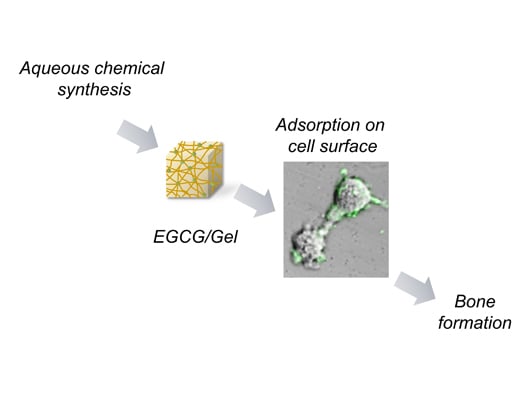
Local Controlled Release of Polyphenol Conjugated with Gelatin Facilitates Bone Formation
Abstract
:1. Introduction
2. Results
2.1. Synthesis of EGCG/Gels

| Sample # | Sample Name | Gelatin (mg) | EGCG (mg) | DMT-MM (mg) | NMM (μL) | Form after Lyophilization | Note |
|---|---|---|---|---|---|---|---|
| 1 | Gel | 100 | 0 | 0 | 0 | Sponge | Readily dissolved in water |
| 2 | – | 100 | 0 | 13.8 | 5.5 | Sponge | Readily dissolved in water |
| 3 | – | 100 | 0.13 | 69.2 | 27.5 | Sponge | Readily dissolved in water |
| 4 | – | 100 | 0.13 | 138 | 55 | Sponge | Readily dissolved in water |
| 5 | EGCG(0.7)/Gel | 100 | 0.7 | 69.2 | 27.5 | Sponge | |
| 6 | – | 100 | 6.7 | 13.8 | 5.5 | Sponge | |
| 7 | EGCG(6.7)/Gel | 100 | 6.7 | 69.2 | 27.5 | Sponge | |
| 8 | – | 100 | 67 | 138 | 55 | Powder |

2.2. Degradation of EGCG/Gels

2.3. Osteogenic Capability of EGCG/Gels


2.4. Effects of EGCG/Gels on Osteoblast Differentiation
2.5. Adsorption of EGCG/Gel to the D1 Cells

3. Discussion
4. Experimental Section
4.1. Chemicals
4.2. Aqueous Chemical Synthesis of EGCG/Gels
4.3. Synthesis of FITC-Labeled EGCG/Gel
4.4. Characterization of Gel and EGCG/Gels
4.5. Robustness of EGCG/Gels
4.6. Animal Experiments
4.7. Cell Maintenance
4.8. Effects of EGCG/Gel for Osteoblastogenesis
4.9. Adsorption of FITC-Labeled-EGCG (0.7)/Gel onto a Mesenchymal Stem Cell Line
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aw, M.S.; Addai-Mensah, J.; Losic, D. Magnetic-responsive delivery of drug-carriers using titania nanotube arrays. J. Mater. Chem. 2012, 22, 6561–6563. [Google Scholar] [CrossRef]
- Gulati, K.; Aw, M.S.; Losic, D. Nanoengineered drug-releasing Ti wires as an alternative for local delivery of chemotherapeutics in the brain. Int. J. Nanomed. 2012, 7, 2069–2076. [Google Scholar]
- Dubin, C. A one-two punch: Drug/medical device combination products are taking healthcare in a new direction. Is the pharmaceutical industry prepared? Drug Deliv. Technol. 2004, 4. Available online: http://drug-dev.com/Main/Back-Issues/A-OneTwo-Punch-DrugMedical-Device-Combination-Prod-241.aspx (accessed on 1 April 2015).
- Kaplish, V. Local drug delivery systems in the treatment of periodontitis: A review. Pharmacophore 2013, 4, 39–42. [Google Scholar]
- Wu, P.; Grainger, D.W. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials 2006, 27, 2450–2467. [Google Scholar] [CrossRef] [PubMed]
- Wildemann, B.; Bamdad, P.; Holmer, C.; Haas, N.P.; Raschke, M.; Schmidmaier, G. Local delivery of growth factors from coated titanium plates increases osteotomy healing in rats. Bone 2004, 34, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, D.Y.; Huang, L. In vivo delivery of miRNAs for cancer therapy: Challenges and strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Munin, A.; Edwards-Levy, F. Encapsulation of natural polyphenolic compounds: A review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Han, J.H.; Hwang, E.J.; Seo, J.Y.; Cho, K.H.; Kim, K.H.; Youn, J.I.; Eun, H.C. Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J. 2003, 17, 1913–1915. [Google Scholar] [PubMed]
- Park, G.; Yoon, B.S.; Moon, J.H.; Kim, B.; Jun, E.K.; Oh, S.; Kim, H.; Song, H.J.; Noh, J.Y.; Oh, C.; et al. Green tea polyphenol epigallocatechin-3-gallate suppresses collagen production and proliferation in keloid fibroblasts via inhibition of the STAT3-signaling pathway. J. Investig. Dermatol. 2008, 128, 2429–2441. [Google Scholar] [CrossRef] [PubMed]
- Takai, S.; Matsushima-Nishiwaki, R.; Adachi, S.; Natsume, H.; Minamitani, C.; Mizutani, J.; Otsuka, T.; Tokuda, H.; Kozawa, O. (−)-Epigallocatechin gallate reduces platelet-derived growth factor-BB-stimulated interleukin-6 synthesis in osteoblasts: Suppression of SAPK/JNK. Mediat. Inflamm. 2008, 2008, 291808. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Suzui, M.; Lim, J.T.; Deguchi, A.; Soh, J.W.; Weinstein, I.B. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J. Exp. Ther. Oncol. 2002, 2, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Sakamoto, K. (−)-Epigallocatechin gallate suppresses adipocyte differentiation through the MEK/ERK and PI3K/Akt pathways. Cell Biol. Int. 2012, 36, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wu, H.; Xu, G.; Zheng, L.; Zhao, J. Epigallocatechin-3-gallate (EGCG) as a pro-osteogenic agent to enhance osteogenic differentiation of mesenchymal stem cells from human bone marrow: An in vitro study. Cell Tissue Res. 2014, 356, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Vali, B.; Rao, L.G.; El-Sohemy, A. Epigallocatechin-3-gallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J. Nutr. Biochem. 2007, 18, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I. Multifunctional effects of green tea catechins on prevention of the metabolic syndrome. Asia Pac. J. Clin. Nutr. 2008, 17, 273–274. [Google Scholar] [PubMed]
- Venkateswara, B.; Sirisha, K.; Chava, V. Green tea extract for periodontal health. J. Indian Soc. Periodontol. 2011, 15, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Kuzuya, M.; Cheng, X.W.; Asai, T.; Kanda, S.; Tamaya-Mori, N.; Sasaki, T.; Shibata, T.; Iguchi, A. Green tea catechins inhibit the cultured smooth muscle cell invasion through the basement barrier. Atherosclerosis 2003, 166, 23–30. [Google Scholar] [CrossRef]
- Rodriguez, R.; Kondo, H.; Nyan, M.; Hao, J.; Miyahara, T.; Ohya, K.; Kasugai, S. Implantation of green tea catechin α-tricalcium phosphate combination enhances bone repair in rat skull defects. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Yeh, J.K.; Cao, J.J.; Wang, J.S. Green tea and bone metabolism. Nutr. Res. 2009, 29, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Bhatnagar, P.; Singh, M.; Mishra, S.; Kumar, P.; Shukla, Y.; Gupta, K.C. Synthesis of PLGA nanoparticles of tea polyphenols and their strong in vivo protective effect against chemically induced DNA damage. Int. J. Nanomed. 2013, 8, 1451–1462. [Google Scholar]
- Yu, S.-H.; Tsai, M.-L.; Lin, B.-X.; Lin, C.-W.; Mi, F.-L. Tea catechins-cross-linked methylcellulose active films for inhibition of light irradiation and lipid peroxidation induced β-carotene degradation. Food Hydrocoll. 2015, 44, 491–505. [Google Scholar] [CrossRef]
- Garcia, J.; Hsieh, M.-F.; Doma, B.; Peruelo, D.; Chen, I.-H.; Lee, H.-M. Synthesis of gelatin-γ-polyglutamic acid-based hydrogel for the in vitro controlled release of epigallocatechin gallate (EGCG) from camellia sinensis. Polymers 2013, 6, 39–58. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Feng, C.-L.; Lai, C.-H.; Lin, J.-H.; Chen, H.-Y. Preparation of epigallocatechin gallate-loaded nanoparticles and characterization of their inhibitory effects on Helicobacter pylori growth in vitro and in vivo. Sci. Technol. Adv. Mater. 2014, 15, 045006. [Google Scholar] [CrossRef]
- Chen, C.H.; Ho, M.L.; Chang, J.K.; Hung, S.H.; Wang, G.J. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos. Int. 2005, 16, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Kamon, M.; Zhao, R.; Sakamoto, K. Green tea polyphenol (−)-epigallocatechin gallate suppressed the differentiation of murine osteoblastic MC3T3-E1 cells. Cell Biol. Int. 2009, 34, 109–116. [Google Scholar] [PubMed]
- Wei, Y.J.; Tsai, K.S.; Lin, L.C.; Lee, Y.T.; Chi, C.W.; Chang, M.C.; Tsai, T.H.; Hung, S.C. Catechin stimulates osteogenesis by enhancing PP2A activity in human mesenchymal stem cells. Osteoporos. Int. 2011, 22, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Mann, J.K.; Kundu, S.C. Silk fibroin/gelatin multilayered films as a model system for controlled drug release. Eur. J. Pharm. Sci. 2009, 37, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Yin, Y.; Xu, M.; Wang, Y. Investigation of pH-sensitive drug-delivery system of chitosan/gelatin hybrid polymer network. Polym. Int. 1995, 38, 77–82. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; van Wachem, P.B.; van Luyn, M.J.; Plantinga, J.A.; Engbers, G.H.; Krijgsveld, J.; Zaat, S.A.; Dankert, J.; Feijen, J. In vivo compatibility and degradation of crosslinked gelatin gels incorporated in knitted Dacron. J. Biomed. Mater. Res. 2000, 51, 136–145. [Google Scholar] [CrossRef]
- Aalami, O.O.; Nacamuli, R.P.; Lenton, K.A.; Cowan, C.M.; Fang, T.D.; Fong, K.D.; Shi, Y.-Y.; Song, H.M.; Sahar, D.E.; Longaker, M.T. Applications of a mouse model of calvarial healing: Differences in regenerative abilities of juveniles and adults. Plast. Reconstr. Surg. 2004, 114, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.A.; O’Brien, F.; Little, D.; Schindeler, A. Cell-scaffold interactions in the bone tissue engineering triad. Eur. Cells Mater. 2013, 26, 120–132. [Google Scholar]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Rani, B.; Maheshwari, R.; Chauhan, A.; Singh, U. Potentiality of green chemistry for future perspectives. Int. J. Pharm. Pharm. Sci. 2012, 1, 97–104. [Google Scholar]
- Kunishima, M.; Kawachi, C.; Morita, J.; Terao, K.; Iwasaki, F.; Tani, S. 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride: An efficient condensing agent leading to the formation of amides and esters. Tetrahedron 1999, 55, 13159–12170. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Collagen- vs. gelatine-based biomaterials and their biocompatibility: Review and perspectives. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; InTech: Rejeka, Croatia, 2011; pp. 17–52. [Google Scholar]
- Johansson, S.; Svineng, G.; Wennerberg, K.; Armulik, A.; Lohikangas, L. Fibronectin-integrin interactions. Front. Biosci. 1997, 2, d126–d146. [Google Scholar] [PubMed]
- Honda, Y.; Anada, T.; Kamakura, S.; Morimoto, S.; Kuriyagawa, T.; Suzuki, O. The effect of microstructure of octacalcium phosphate on the bone regenerative property. Tissue Eng. Part A 2009, 15, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Hsiong, S.X.; Boontheekul, T.; Huebsch, N.; Mooney, D.J. Cyclic arginine-glycine-aspartate peptides enhance three-dimensional stem cell osteogenic differentiation. Tissue Eng. Part A 2009, 15, 263–272. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honda, Y.; Tanaka, T.; Tokuda, T.; Kashiwagi, T.; Kaida, K.; Hieda, A.; Umezaki, Y.; Hashimoto, Y.; Imai, K.; Matsumoto, N.; et al. Local Controlled Release of Polyphenol Conjugated with Gelatin Facilitates Bone Formation. Int. J. Mol. Sci. 2015, 16, 14143-14157. https://doi.org/10.3390/ijms160614143
Honda Y, Tanaka T, Tokuda T, Kashiwagi T, Kaida K, Hieda A, Umezaki Y, Hashimoto Y, Imai K, Matsumoto N, et al. Local Controlled Release of Polyphenol Conjugated with Gelatin Facilitates Bone Formation. International Journal of Molecular Sciences. 2015; 16(6):14143-14157. https://doi.org/10.3390/ijms160614143
Chicago/Turabian StyleHonda, Yoshitomo, Tomonari Tanaka, Tomoko Tokuda, Takahiro Kashiwagi, Koji Kaida, Ayato Hieda, Yasuyuki Umezaki, Yoshiya Hashimoto, Koichi Imai, Naoyuki Matsumoto, and et al. 2015. "Local Controlled Release of Polyphenol Conjugated with Gelatin Facilitates Bone Formation" International Journal of Molecular Sciences 16, no. 6: 14143-14157. https://doi.org/10.3390/ijms160614143
APA StyleHonda, Y., Tanaka, T., Tokuda, T., Kashiwagi, T., Kaida, K., Hieda, A., Umezaki, Y., Hashimoto, Y., Imai, K., Matsumoto, N., Baba, S., & Shimizutani, K. (2015). Local Controlled Release of Polyphenol Conjugated with Gelatin Facilitates Bone Formation. International Journal of Molecular Sciences, 16(6), 14143-14157. https://doi.org/10.3390/ijms160614143