β-Radiation Stress Responses on Growth and Antioxidative Defense System in Plants: A Study with Strontium-90 in Lemna minor
Abstract
:1. Introduction
2. Results
2.1. 90Sr Uptake and Dosimetry

| Activity Concentration | Biomass | Uptake Parameters | Dosimetric Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Nominal [kBq·L−1] | Measured [kBq·L−1] | FW [mg] | DW [mg] | Conc. Ratio FW [Bq·kg−1 FW/Bq·L−1] | Conc. Ratio DW [Bq·kg−1 DW/Bq·L−1] | Dose Rate Intern [µGy·h−1] | Dose Rate Extern [µGy·h−1] | Total Dose Rate [µGy·h−1] | |
| 0 | 0 ± 0.001 | plant | 199.5 ± 10 | 13.9 ± 0.7 | na | na | na | na | na |
| frond | 165.6 ± 7.8 | 12.3 ± 0.6 | na | na | na | na | na | ||
| root | 33.8 ± 2.3 | 1.6 ± 0.1 | na | na | na | na | na | ||
| 25 | 25 ± 0.1 | plant | 225.3 ± 10.4 | 15.3 ± 0.5 | 60 ± 3 | 832 ± 21 | 85 ± 4 | 5 ± 0.2 | 84 ± 4 |
| frond | 186 ± 8.8 | 13.5 ± 0.3 | 62 ± 2 | 829 ± 30 | 86 ± 4 | 5 ± 0.2 | 89 ± 4 | ||
| root | 38.8 ± 1.8 | 1.8 ± 0.2 | 28 ± 2 | 783 ± 23 | 41 ± 3 | 10 ± 0.4 | 51 ± 2 | ||
| 250 | 247 ± 0.1 | plant | 210.2 ± 9.6 | 15.1 ± 0.9 | 60 ± 4 | 767 ± 65 | 918 ± 63 | 53 ± 3 | 967 ± 58 |
| frond | 178 ± 8 | 13.7 ± 0.7 | 65 ± 5 | 789 ± 71 | 1000 ± 76 | 53 ± 3 | 1053 ± 74 | ||
| root | 31.2 ± 1.6 | 1.5 ± 0.1 | 29 ± 1 | 752 ± 15 | 454 ± 20 | 107 ± 5 | 562 ± 21 | ||
| 2500 | 2453 ± 2 | plant | 195.6 ± 5.6 | 14.0 ± 0.2 | 60 ± 3 | 844 ± 23 | 8881 ± 486 | 530 ± 3 | 9432 ± 483 |
| frond | 178.5 ± 5 | 13.2 ± 0.3 | 63 ± 3 | 858 ± 24 | 9185 ± 477 | 530 ± 3 | 9715 ± 476 | ||
| root | 16.8 ± 0.7 * | 0.9 ± 0.1 * | 37 ± 3 | 820 ± 48 | 5486 ± 469 | 1070 ± 6 | 6556 ± 464 | ||
| 25,000 | 24,567 ± 13 | plant | 124.4 ± 3.4 | 12.5 ± 0.1 | 57 ± 5 | 540 ± 35 * | 91,063 ± 8607 | 6006 ± 98 | 97,264 ± 8490 |
| frond | 118.7 ± 3.5 * | 12.2 ± 0.1 | 59 ± 6 | 542 ± 36 * | 92,827 ± 9130 | 6006 ± 98 | 98,834 ± 9033 | ||
| root | 5.7 ± 0.2 * | 0.4 ± 0.02 * | 36 ± 2 | 474 ± 20 * | 56,904 ± 2287 | 12,121 ± 197 | 69,025 ± 2181 | ||
2.2. 90Sr Induced Effects in L. minor
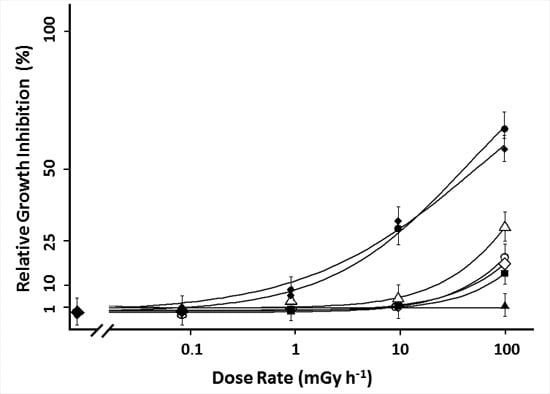
 ), frond number (
), frond number (  ), plant fresh weight (
), plant fresh weight (  ), frond fresh weight (
), frond fresh weight (  ), frond dry weight (
), frond dry weight (  ), root fresh weight (
), root fresh weight (  ) and root dry weight (
) and root dry weight (  ). Each data point represents the mean ± SE of nine biological replicas for frond related endpoints and three biological replicas for root related endpoints. In addition to the average values of each point, the log-logistic function fitted through the data is added.
). Each data point represents the mean ± SE of nine biological replicas for frond related endpoints and three biological replicas for root related endpoints. In addition to the average values of each point, the log-logistic function fitted through the data is added.
 ), frond number (
), frond number (  ), plant fresh weight (
), plant fresh weight (  ), frond fresh weight (
), frond fresh weight (  ), frond dry weight (
), frond dry weight (  ), root fresh weight (
), root fresh weight (  ) and root dry weight (
) and root dry weight (  ). Each data point represents the mean ± SE of nine biological replicas for frond related endpoints and three biological replicas for root related endpoints. In addition to the average values of each point, the log-logistic function fitted through the data is added.
). Each data point represents the mean ± SE of nine biological replicas for frond related endpoints and three biological replicas for root related endpoints. In addition to the average values of each point, the log-logistic function fitted through the data is added.


3. Discussion
3.1. 90Sr Accumulation and Uptake
3.2. Dosimetry
3.3. Antioxidative Response
4. Experimental Section
4.1. Culture Stock
4.2. 90Sr Exposure
4.3. 90Sr Transfer and Dosimetry
4.4. Plant Growth
4.5. Antioxidative Enzyme Activities
4.6. Metabolite Measurements
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bradley, E.S. Radionuclides in biota. In Environmental Contaminants in Biota; CRC Press: Boca Raton, FL, USA, 2011; pp. 703–732. [Google Scholar]
- Roussel-Debet, S.; Beaugelin, K. Strontium 90 et Environnement; IRSN: Paris, France, 2005. [Google Scholar]
- Buesseler, K.O.; Livingston, H.D. Natural and man-made radionuclides in the black sea. In Adionuclides in the Oceans, Inputs and Inventories; Guéguéniat, P., Germain, P., Métivier, H., Eds.; Institut de Protection et de Surete Nucleaire: Cherbourg, France, 1996; pp. 199–217. [Google Scholar]
- Yu, W.; He, J.; Lin, W.; Li, Y.; Men, W.; Wang, F.; Huang, J. Distribution and risk assessment of radionuclides released by fukushima nuclear accident at the northwest pacific. J. Environ. Radioact. 2015, 142C, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Comans, R.N.; Beresford, N.A.; Wright, S.M.; Howard, B.J.; Camplin, W.C. Chernobyl’s legacy in food and water. Nature 2000, 405, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzoyeva, N.Y.; Egorov, V.N.; Polikarpov, G.G. Distribution and migration of (9)(0)sr in components of the dnieper river basin and the black sea ecosystems after the chernobyl npp accident. J. Environ. Radioact. 2013, 125, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Penrose, B.; Beresford, N.A.; Broadley, M.R.; Crout, N.M.J. Inter-varietal variation in caesium and strontium uptake by plants: A meta-analysis. J. Environ. Radioact. 2015, 139, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Scotti, I.A.; Carini, F. Heavy metal effect on uptake and translocation of 134cs and 85sr in aubergine plants. J. Environ. Radioact. 2000, 48, 183–190. [Google Scholar] [CrossRef]
- Baeza, A.; Paniagua, J.M.; Rufo, M.; Sterling, A.; Barandica, J. Radiocaesium and radiostrontium uptake by turnips and broad beans via leaf and root absorption. Appl. Radiat. Isot. 1999, 50, 467–474. [Google Scholar] [CrossRef]
- Kozhevnikova, A.D.; Seregin, I.V.; Bystrova, E.I.; Belyaeva, A.I.; Kataeva, M.N.; Ivanov, V.B. The effects of lead, nickel, and strontium nitrates on cell division and elongation in maize roots. Russ. J. Plant Physiol. 2009, 56, 242–250. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J. Some elements are more equal than others: Soil-to-plant transfer of radiocaesium and radiostrontium, revisited. Plant Soil 2012, 355, 23–27. [Google Scholar] [CrossRef]
- Camps, M.; Rigol, A.; Vidal, M.; Rauret, G. Assessment of the suitability of soil amendments to reduce cs-137 and sr-90 root uptake in meadows. Environ. Sci. Technol. 2003, 37, 2820–2828. [Google Scholar] [CrossRef] [PubMed]
- Dan, W.; Fangping, W.; Changhe, X.; Yunlai, T.; Xuegang, L. The uptake of cs and sr from soil to radish (raphanus sativus l.)-potential for phytoextraction and remediation of contaminated soils. J. Environ. Radioact. 2012, 110, 78–83. [Google Scholar]
- Willey, N.; Fawcett, K. A phylogenetic effect on strontium concentrations in angiosperms. Environ. Exp. Bot. 2006, 57, 258–269. [Google Scholar] [CrossRef]
- Chen, M.; Tang, Y.L.; Ao, J.; Wang, D. Effects of strontium on photosynthetic characteristics of oilseed rape seedlings. Russ. J. Plant Physiol. 2012, 59, 772–780. [Google Scholar] [CrossRef]
- Kanter, U.; Hauser, A.; Michalke, B.; Draxl, S.; Schaffner, A.R. Caesium and strontium accumulation in shoots of arabidopsis thaliana: Genetic and physiological aspects. J. Exp. Bot. 2010, 61, 3995–4009. [Google Scholar] [CrossRef] [PubMed]
- Moyen, C.; Roblin, G. Uptake and translocation of strontium in hydroponically grown maize plants, and subsequent effects on tissue ion content, growth and chlorophyll a/b ratio: Comparison with ca effects. Environ. Exp. Bot. 2010, 68, 247–257. [Google Scholar] [CrossRef]
- Copplestone, D.; Brown, J.E.; Beresford, N.A. Considerations for the integration of human and wildlife radiological assessments. J. Radiol. Prot. 2010, 30, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Biermans, G.; Horemans, N.; Vanhoudt, N.; Vandenhove, H.; Saenen, E.; van Hees, M.; Wannijn, J.; Vives i Batlle, J.; Cuypers, A. An organ-based approach to dose calculation in the assessment of dose-dependent biological effects of ionising radiation in arabidopsis thaliana. J. Environ. Radioact. 2014, 133, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Hunter, N.; Muirhead, C.R. Review of relative biological effectiveness dependence on linear energy transfer for low-let radiations. J. Radiol. Prot. 2009, 29, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.E.; Hwang, S.-G.; Kim, S.-H.; Lee, K.J.; Jang, C.S.; Kim, J.-B.; Kim, S.H.; Ha, B.-K.; Ahn, J.-W.; Kang, S.-Y.; et al. Transcriptome profiling in response to different types of ionizing radiation and identification of multiple radio marker genes in rice. Physiol. Plant. 2014, 150, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Horemans, N.; Foyer, C.H.; Potters, G.; Asard, H. Ascorbate function and associated transport systems in plants. Plant Physiol. Biochem. 2000, 38, 531–540. [Google Scholar] [CrossRef]
- Tkalec, M.; Malaric, K.; Pevalek-Kozlina, B. Exposure to radiofrequency radiation induces oxidative stress in duckweed lemna minor l. Sci. Total Environ. 2007, 388, 78–89. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Arena, C.; Pignalosa, D.; Durante, M. Effects of sparsely and densely ionizing radiation on plants. Radiat. Environ. Biophys. 2011, 50, 1–19. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, T.; Chawla, F.; Steinmann, P.; Gobat, J.M.; Froidevaux, P. Disparity in sr-90 and cs-137 uptake in alpine plants: Phylogenetic effect and ca and k availability. Plant Soil 2012, 355, 29–39. [Google Scholar] [CrossRef]
- Zeller, S.; Feller, U. Long-distance transport of alkali metals in maturing wheat. Biol. Plant. 2000, 43, 523–528. [Google Scholar] [CrossRef]
- White, P.J. The pathways of calcium movement to the xylem. J. Exp. Bot. 2001, 52, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Mazen, A.M.A.; Zhang, D.Z.; Franceschi, V.R. Calcium oxalate formation in lemna minor: Physiological and ultrastructural aspects of high capacity calcium sequestration. New Phytol. 2004, 161, 435–448. [Google Scholar] [CrossRef]
- Franceschi, V.R. Calcium-oxalate formation is a rapid and reversible process in lemna-minor-l. Protoplasma 1989, 148, 130–137. [Google Scholar] [CrossRef]
- OECD. Lemna sp. Growth Inhibition Test; Guideline 221; Organisation for Economic Co-operation and Development: Paris, France, 2006. [Google Scholar]
- Brain, R.A.; Solomon, K.R. A protocol for conducting 7-day daily renewal tests with lemna gibba. Nat. Protoc. 2007, 2, 979–987. [Google Scholar] [CrossRef] [PubMed]
- UNSCEAR. Effects of Ionizing Radiation United Nations Scientific Committee on the Effects of Atomic Radiation: Unscear 2008 Report to the General Assembly, with Scientific Annexes; United Nations: New York, NY, USA, 2011. [Google Scholar]
- Anamika, S.; Eapen, S.; Fulekar, M.H. Phytoremediation technology for remediation of radiostrontium (90sr) and radiocesium (137cs) by catharanthus roseus (L.) G. Don in aquatic environment. Environ. Eng. Manag. J. 2009, 8, 527–532. [Google Scholar]
- Soudek, P.; Valenová, Š.; Vavříková, Z.; Vaněk, T. 137cs and 90sr uptake by sunflower cultivated under hydroponic conditions. J. Environ. Radioact. 2006, 88, 236–250. [Google Scholar] [CrossRef] [PubMed]
- IAEA. Handbook of Parameter Values for the Prediction of Radionuclide Transfer in Terrestrial and Freshwater Environments; Technical Reports Series No. 472; IAEA: Vienna, Austria, 2010. [Google Scholar]
- Brown, J.E.; Alfonso, B.; Avila, R.; Beresford, N.A.; Copplestone, D.; Prohl, G.; Ulanovsky, A. The erica tool. J. Environ. Radioact. 2008, 99, 1371–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadzis, D.R.; Leinerte, M.P.; Seisuma, Z.K.; Sloka, Y.Y. Strontium and Calcium in Natural Freshwater Ecosystemsl; Riga Zinatne: Latvia, 1979. [Google Scholar]
- Yankovich, T.L.; Vives i Batlle, J.; Vives-Lynch, S.; Beresford, N.A.; Barnett, C.L.; Beaugelin-Seiller, K.; Brown, J.E.; Cheng, J.J.; Copplestone, D.; Heling, R.; et al. An international model validation exercise on radionuclide transfer and doses to freshwater biota. J. Radiol. Prot. 2010, 30, 299–340. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Kim, Y.J.; Choi, E.M.; Brown, M.T.; Han, T. A novel bioassay using root re-growth in lemna. Aquat. Toxicol. 2013, 140–141, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Gopalapillai, Y.; Vigneault, B.; Hale, B.A. Root length of aquatic plant, lemna minor l., as an optimal toxicity endpoint for biomonitoring of mining effluents. Integr. Environ. Assess. Manag. 2014, 10, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Arts, G.H.P.; Belgers, J.D.M.; Hoekzema, C.H.; Thissen, J. Sensitivity of submersed freshwater macrophytes and endpoints in laboratory toxicity tests. Environ. Pollut. 2008, 153, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Copplestone, D.; Hingston, J.; Real, A. The development and purpose of the frederica radiation effects database. J. Environ. Radioact. 2008, 99, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Garnier-Laplace, J.; Copplestone, D.; Gilbin, R.; Alonzo, F.; Ciffroy, P.; Gilek, M.; Aguero, A.; Bjork, M.; Oughton, D.H.; Jaworska, A.; et al. Issues and practices in the use of effects data from frederica in the erica integrated approach. J. Environ. Radioact. 2008, 99, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, D.I.; Mardarevich, M.G.; Kipnis, L.S.; Ponomaryov, A.V. Long-term combined impact of 90sr and pb2+ on freshwater cladoceran. In Protection of the Environment From Ionising Radiation. Proceedings of the Third International Symposium on the Protection of the Environment from Ionising Radiation (SPEIR 3), Darwin, Australia, 22–26 July 2002; Volume 34, pp. 43–49.
- Amiro, B.D. Response of boreal forest tree canopy cover to chronic gamma-irradiation. J. Environ. Radioact. 1994, 24, 181–197. [Google Scholar] [CrossRef]
- UNSCEAR. Sources and effects of ionizing radiation. In UNSCEAR 1996 Report to the General Assembly, with Scientific Annex; United Nations Scientific Committee on the Effects of Atomic Radiation: New York, NY, USA, 1996; p. 86. [Google Scholar]
- Garnier-Laplace, J.; Geras'kin, S.; Della-Vedova, C.; Beaugelin-Seiller, K.; Hinton, T.G.; Real, A.; Oudalova, A. Are radiosensitivity data derived from natural field conditions consistent with data from controlled exposures? A case study of chernobyl wildlife chronically exposed to low dose rates. J. Environ. Radioact. 2013, 121, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, I.; Molinier, J.; Yao, Y.L.; Arkhipov, A.; Kovalchuk, O. Transcriptome analysis reveals fundamental differences in plant response to acute and chronic exposure to ionizing radiation. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2007, 624, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Gicquel, M.; Taconnat, L.; Renou, J.P.; Esnault, M.A.; Cabello-Hurtado, F. Kinetic transcriptomic approach revealed metabolic pathways and genotoxic-related changes implied in the arabidopsis response to ionising radiations. Plant Sci. 2012, 195, 106–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.-J.; Cheng, T.-S. Oxidative effects and metabolic changes following exposure of greater duckweed (spirodela polyrhiza) to diethyl phthalate. Aquat. Toxicol. 2012, 109, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Kim, Y.-S.; Hahn, K.-W.; Joung, H.; Jeon, J.-H. Chapter 2 reactive oxygen species: Regulation of plant growth and developmen. In Advances in Botanical Research; Jean-Pierre, J., Ed.; Academic Press: Waltham, MA, USA, 2009; Volume 52, pp. 25–46. [Google Scholar]
- Noctor, G.; Gomez, L.; Vanacker, H.; Foyer, C.H. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J. Exp. Bot. 2002, 53, 1283–1304. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, J.; Pflugmacher, S. Ammonia triggers the promotion of oxidative stress in the aquatic macrophyte myriophyllum mattogrossense. Chemosphere 2007, 66, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Hou, J.; Li, W.; Zhang, W.J. Metabolic adaptations to ammonia-induced oxidative stress in leaves of the submerged macrophyte vallisneria natans (lour.) hara. Aquat. Toxicol. 2008, 87, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chung, B.Y.; Kim, J.S.; Wi, S.G. Effects of in planta gamma-irradiation on growth, photosynthesis, and antioxidative capacity of red pepper (capsicum annuum l.) plants. J. Plant Biol. 2005, 48, 47–56. [Google Scholar] [CrossRef]
- Vanhoudt, N.; Vandenhove, H.; Horemans, N.; Wannijn, J.; van Hees, M.; Vangronsveld, J.; Cuypers, A. The combined effect of uranium and gamma radiation on biological responses and oxidative stress induced in arabidopsis thaliana. J. Environ. Radioact. 2010, 101, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Zaka, R.; Vandecasteele, C.M.; Misset, M.T. Effects of low chronic doses of ionizing radiation on antioxidant enzymes and g(6)pdh activities in stipa capillata (poaceae). J. Exp. Bot. 2002, 53, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Cedergreen, N.; Abbaspoor, M.; Sorensen, H.; Streibig, J.C. Is mixture toxicity measured on a biomarker indicative of what happens on a population level? A study with lemna minor. Ecotoxicol. Environ. Saf. 2007, 67, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, R. Mineral requirement of lemna minor. Plant Physiol. 1946, 21, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image Processing Using Imagej. Biophotonics International; Laurin Publishing Co., Inc.: Pittsfield, MA, USA, 2004. [Google Scholar]
- Lemon, G.D.; Posluszny, U.; Husband, B.C. Potential and realized rates of vegetative reproduction in spirodela polyrhiza, lemna minor, and wolffia borealis. Aquat. Bot. 2001, 70, 79–87. [Google Scholar] [CrossRef]
- Ziegler, P.; Adelmann, K.; Zimmer, S.; Schmidt, C.; Appenroth, K.J. Relative in vitro growth rates of duckweeds (lemnaceae)—The most rapidly growing higher plants. Plant Biol. 2015, 17 (Suppl. 1), 33–41. [Google Scholar] [CrossRef] [PubMed]
- Horemans, N.; van Hees, M.; van Hoeck, A.; Saenen, E.; de Meutter, T.; Nauts, R.; Blust, R.; Vandenhove, H. Uranium and cadmium provoke different oxidative stress responses in lemna minor l. Plant Biol. 2015, 17 (Suppl. 1), 91–100. [Google Scholar] [CrossRef] [PubMed]
- Queval, G.; Noctor, G. A plate reader method for the measurement of nad, nadp, glutathione, and ascorbate in tissue extracts: Application to redox profiling during arabidopsis rosette development. Anal. Biochem. 2007, 363, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Streibig, J.C. Bioassay analysis using r. J. Stat. Soft. 2005, 12, 1–22. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Hoeck, A.; Horemans, N.; Van Hees, M.; Nauts, R.; Knapen, D.; Vandenhove, H.; Blust, R. β-Radiation Stress Responses on Growth and Antioxidative Defense System in Plants: A Study with Strontium-90 in Lemna minor. Int. J. Mol. Sci. 2015, 16, 15309-15327. https://doi.org/10.3390/ijms160715309
Van Hoeck A, Horemans N, Van Hees M, Nauts R, Knapen D, Vandenhove H, Blust R. β-Radiation Stress Responses on Growth and Antioxidative Defense System in Plants: A Study with Strontium-90 in Lemna minor. International Journal of Molecular Sciences. 2015; 16(7):15309-15327. https://doi.org/10.3390/ijms160715309
Chicago/Turabian StyleVan Hoeck, Arne, Nele Horemans, May Van Hees, Robin Nauts, Dries Knapen, Hildegarde Vandenhove, and Ronny Blust. 2015. "β-Radiation Stress Responses on Growth and Antioxidative Defense System in Plants: A Study with Strontium-90 in Lemna minor" International Journal of Molecular Sciences 16, no. 7: 15309-15327. https://doi.org/10.3390/ijms160715309
APA StyleVan Hoeck, A., Horemans, N., Van Hees, M., Nauts, R., Knapen, D., Vandenhove, H., & Blust, R. (2015). β-Radiation Stress Responses on Growth and Antioxidative Defense System in Plants: A Study with Strontium-90 in Lemna minor. International Journal of Molecular Sciences, 16(7), 15309-15327. https://doi.org/10.3390/ijms160715309