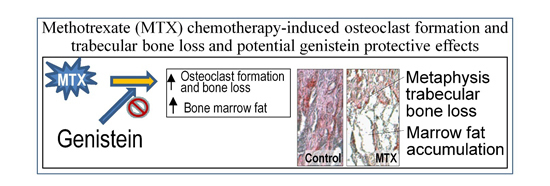
Potential Effects of Phytoestrogen Genistein in Modulating Acute Methotrexate Chemotherapy-Induced Osteoclastogenesis and Bone Damage in Rats
Abstract
:1. Introduction
2. Results
2.1. Effects on Body Weight Changes

2.2. Effects on Bone Volume and Trabecular Structure and Bone Turnover

2.3. Effects on Densities of Bone Modelling/Remodeling Cells within the Metaphysis

2.4. Effects on ex Vivo Osteoclastogenesis

2.5. Effects on Expression of Osteoclastogenesis-Related Genes in Metaphyseal Bone

2.6. Bone Marrow Adiposity

3. Discussion
4. Experimental Section
4.1. Animal Trials with Methotrexate (MTX) and/or Genistein Administration
4.2. Histological Analyses of Bone Volume, Trabecular Architecture, and Cell Densities
4.3. Serum Alkaline Phosphotase (ALP) Concentration
4.4. Ex Vivo Osteoclastogenic Formation
4.5. RNA Isolation and RT-PCR Gene Expression Analyses
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Cyc A | CGTTGGATGGCACGCCTGTG | TGCTGGTCTTGCCATTCCTG |
| RANKL | TGGGCCAAGATCTCTAACATCAC | TGGGCCAAGATCTCTAACATCAC |
| OPG | AGCTGGCACACGAGTGATGAA | CACATTCGCACACTCGGTTGT |
| TNF-α | TGGGCCAAGATCTCTAACATCAC | CTCCGCTTGGTGGTTTGCTACGAC |
| IL-1β | GTTTCCCTCCCTGCCTCTGACA | GACAATGCTGCCTCGTGACC |
| IL-6 | GATACCCACAACAGACCAG | GCCATTGCACAACTCTTTTCTC |
| C/EBPα | TCGCCATGCCGGGAGAACTCTAAC | CTGGAGGTGGCTGCTCATCGGGG |
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arikoski, P.; Komulainen, J.; Riikonen, P.; Parviainen, M.; Jurvelin, J.S.; Voutilainen, R.; Kroger, H. Impaired development of bone mineral density during chemotherapy: A prospective analysis of 46 children newly diagnosed with cancer. J. Bone Miner. Res. 1999, 14, 2002–2009. [Google Scholar] [CrossRef] [PubMed]
- Gnudi, S.; Butturini, L.; Ripamonti, C.; Avella, M.; Bacci, G. The effects of methotrexate (MTX) on bone. A densitometric study conducted on 59 patients with mtx administered at different doses. Ital. J. Orthop. Traumatol. 1988, 14, 227–231. [Google Scholar] [PubMed]
- Halton, J.M.; Atkinson, S.A.; Fraher, L.; Webber, C.; Gill, G.J.; Dawson, S.; Barr, R.D. Altered mineral metabolism and bone mass in children during treatment for acute lymphoblastic leukemia. J. Bone Miner. Res. 1996, 11, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Pfeilschifter, J.; Diel, I.J. Osteoporosis due to cancer treatment: Pathogenesis and management. J. Clin. Oncol. 2000, 18, 1570–1593. [Google Scholar] [PubMed]
- Hadji, P.; Ziller, M.; Maskow, C.; Albert, U.; Kalder, M. The influence of chemotherapy on bone mineral density, quantitative ultrasonometry and bone turnover in pre-menopausal women with breast cancer. Eur. J. Cancer 2009, 45, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Haddy, T.B.; Mosher, R.B.; Reaman, G.H. Osteoporosis in survivors of acute lymphoblastic leukemia. Oncologist 2001, 6, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.H.; Evans, B.A.; Gregory, J.W. Bone mass acquisition in healthy children. Arch. Dis. Child. 2005, 90, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Mandel, K.; Atkinson, S.; Barr, R.D.; Pencharz, P. Skeletal morbidity in childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2004, 22, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Guise, T.A. Bone loss and fracture risk associated with cancer therapy. Oncologist 2006, 11, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Athanassiadou, F.; Tragiannidis, A.; Rousso, I.; Katsos, G.; Sidi, V.; Papageorgiou, T.; Papastergiou, C.; Tsituridis, I.; Koliouskas, D. Bone mineral density in survivors of childhood acute lymphoblastic leukemia. Turk. J. Pediatr. 2006, 48, 101–104. [Google Scholar] [PubMed]
- Arikoski, P.; Komulainen, J.; Voutilainen, R.; Riikonen, P.; Parviainen, M.; Tapanainen, P.; Knip, M.; Kroger, H. Reduced bone mineral density in long-term survivors of childhood acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 1998, 20, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Jolivet, J.; Cowan, K.H.; Curt, G.A.; Clendeninn, N.J.; Chabner, B.A. The pharmacology and clinical use of methotrexate. N. Engl. J. Med. 1983, 309, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Crofton, P.M.; Ahmed, S.F.; Wade, J.C.; Stephen, R.; Elmlinger, M.W.; Ranke, M.B.; Kelnar, C.J.; Wallace, W.H. Effects of intensive chemotherapy on bone and collagen turnover and the growth hormone axis in children with acute lymphoblastic leukemia. J. Clin. Endocrinol. Metab. 1998, 83, 3121–3129. [Google Scholar] [CrossRef] [PubMed]
- Lequin, M.H.; van der Shuis, I.M.; van Rijn, R.R.; Hop, W.C.; van ven Huevel-Eibrink, M.M.; MuinckKeizer-Schrama, S.M.; van Kuijk, C. Bone mineral assessment with tibial ultrasonometry and dual-energy X-ray absorptiometry in long-term survivors of acute lymphoblastic leukemia in childhood. J. Clin. Densitom. 2002, 5, 167–173. [Google Scholar] [CrossRef]
- Crofton, P.M.; Ahmed, S.F.; Wade, J.C.; Elmlinger, M.W.; Ranke, M.B.; Kelnar, C.J.; Wallace, W.H. Bone turnover and growth during and after continuing chemotherapy in children with acute lymphoblastic leukemia. Pediatr. Res. 2000, 48, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Kaste, S.C.; Jones-Wallace, D.; Rose, S.R.; Boyett, J.M.; Lustig, R.H.; Rivera, G.K.; Pui, C.H.; Hudson, M.M. Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: Frequency of occurrence and risk factors for their development. Leukemia 2001, 15, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Holzer, G.; Krepler, P.; Koschat, M.A.; Grampp, S.; Dominkus, M.; Kotz, R. Bone mineral density in long-term survivors of highly malignant osteosarcoma. J. Bone Jt. Surg. Br. 2003, 85, 231–237. [Google Scholar] [CrossRef]
- Friedlaender, G.E.; Tross, R.B.; Doganis, A.C.; Kirkwood, J.M.; Baron, R. Effects of chemotherapeutic agents on bone. I. Short-term methotrexate and doxorubicin (adriamycin) treatment in a rat model. J. Bone Jt. Surg. Am. 1984, 66, 602–607. [Google Scholar]
- Wheeler, D.L.; vander Griend, R.A.; Wronski, T.J.; Miller, G.J.; Keith, E.E.; Graves, J.E. The short- and long-term effects of methotrexate on the rat skeleton. Bone 1995, 16, 215–221. [Google Scholar] [CrossRef]
- Fan, C.; Cool, J.C.; Scherer, M.A.; Foster, B.K.; Shandala, T.; Tapp, H.; Xian, C.J. Damaging effects of chronic low-dose methotrexate usage on primary bone formation in young rats and potential protective effects of folinic acid supplementary treatment. Bone 2009, 44, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Georgiou, K.R.; King, T.J.; Xian, C.J. Methotrexate toxicity in growing long bones of young rats: A model for studying cancer chemotherapy-induced bone growth defects in children. J. Biomed. Biotechnol. 2011, 2011, 903097. [Google Scholar] [CrossRef] [PubMed]
- Xian, C.J.; Cool, J.C.; Scherer, M.A.; Macsai, C.E.; Fan, C.; Covino, M.; Foster, B.K. Cellular mechanisms for methotrexate chemotherapy-induced bone growth defects. Bone 2007, 41, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, K.R.; King, T.J.; Scherer, M.A.; Zhou, H.; Foster, B.K.; Xian, C.J. Attenuated Wnt/β-catenin signalling mediates methotrexate chemotherapy-induced bone loss and marrow adiposity in rats. Bone 2012, 50, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, K.R.; Scherer, M.A.; Fan, C.M.; Cool, J.C.; King, T.J.; Foster, B.K.; Xian, C.J. Methotrexate chemotherapy reduces osteogenesis but increases adipogenesis potential in the bone marrow. J. Cell. Physiol. 2012, 227, 909–918. [Google Scholar] [CrossRef] [PubMed]
- King, T.J.; Georgiou, K.R.; Cool, J.C.; Scherer, M.A.; Ang, E.S.; Foster, B.K.; Xu, J.; Xian, C.J. Methotrexate chemotherapy promotes osteoclast formation in the long bone of rats via increased pro-inflammatory cytokines and enhanced nf-kappab activation. Am. J. Pathol. 2012, 181, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Leitzel, K.; Ali, S.; Lipton, A. Antiresorptive therapy in the management of cancer treatment-induced bone loss. Curr. Osteoporos. Rep. 2015, 13, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Domschke, C.; Schuetz, F. Side effects of bone-targeted therapies in advanced breast cancer. Breast Care 2014, 9, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Boquete-Castro, A.; Gomez-Moreno, G.; Calvo-Guirado, J.L.; Aguilar-Salvatierra, A.; Delgado-Ruiz, R.A. Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin. Oral Implants Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Xian, C.J.; Cool, J.C.; Scherer, M.A.; Fan, C.; Foster, B.K. Folinic acid attenuates methotrexate chemotherapy-induced damages on bone growth mechanisms and pools of bone marrow stromal cells. J. Cell. Physiol. 2008, 214, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Raghu Nadhanan, R.; Skinner, J.; Chung, R.; Su, Y.W.; Howe, P.R.; Xian, C.J. Supplementation with fish oil and genistein, individually or in combination, protects bone against the adverse effects of methotrexate chemotherapy in rats. PLoS ONE 2013, 8, e71592. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.Y.; Tsai, K.S.; Tu, S.T.; Wu, J.S.; Chang, C.I.; Chen, C.L.; Shaw, N.S.; Peng, H.Y.; Wang, S.Y.; Wu, C.H. The effect of soy isoflavone on bone mineral density in postmenopausal taiwanese women with bone loss: A 2-year randomized double-blind placebo-controlled study. Osteoporos. Int. 2012, 23, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Lydeking-Olsen, E. Dietary phytoestrogens and their effect on bone: Evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am. J. Clin. Nutr. 2003, 78, 593S–609S. [Google Scholar] [PubMed]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: A randomized trial. Ann. Intern. Med. 2007, 146, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Morabito, N.; Crisafulli, A.; Vergara, C.; Gaudio, A.; Lasco, A.; Frisina, N.; DʼAnna, R.; Corrado, F.; Pizzoleo, M.A.; Cincotta, M.; et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: A randomized double-blind placebo-controlled study. J. Bone Miner. Res. 2002, 17, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Hertrampf, T.; Schleipen, B.; Offermanns, C.; Velders, M.; Laudenbach, U.; Diel, P. Comparison of the bone protective effects of an isoflavone-rich diet with dietary and subcutaneous administrations of genistein in ovariectomized rats. Toxicol. Lett. 2009, 184, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Burnett, B.P.; Polito, F.; Marini, H.; Levy, R.M.; Armbruster, M.A.; Minutoli, L.; di Stefano, V.; Irrera, N.; Antoci, S.; et al. Effects of genistein aglycone in osteoporotic, ovariectomized rats: A comparison with alendronate, raloxifene and oestradiol. Br. J. Pharmacol. 2008, 155, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yu, S. Genistein prevents bone resorption diseases by inhibiting bone resorption and stimulating bone formation. Biol. Pharma. Bull. 2003, 26, 780–786. [Google Scholar] [CrossRef]
- Ming, L.G.; Chen, K.M.; Xian, C.J. Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. J. Cell. Physiol. 2013, 228, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Polito, F.; Burnett, B.; Levy, R.; di Stefano, V.; Armbruster, M.A.; Marini, H.; Minutoli, L.; Altavilla, D.; Squadrito, F. Protective effect of genistein aglycone on the development of osteonecrosis of the femoral head and secondary osteoporosis induced by methylprednisolone in rats. J. Endocrinol. 2009, 201, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Gao, Y.H. Inhibitory effect of genistein on bone resorption in tissue culture. Biochem. Pharmacol. 1998, 55, 71–76. [Google Scholar] [CrossRef]
- Marini, H.; Bitto, A.; Altavilla, D.; Burnett, B.P.; Polito, F.; di Stefano, V.; Minutoli, L.; Atteritano, M.; Levy, R.M.; DʼAnna, R.; et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: A follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 4787–4796. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J. Bone Miner. Res. 2008, 23, 715–720. [Google Scholar] [CrossRef] [PubMed]
- McClain, R.M.; Wolz, E.; Davidovich, A.; Pfannkuch, F.; Edwards, J.A.; Bausch, J. Acute, subchronic and chronic safety studies with genistein in rats. Food Chem. Toxicol. 2006, 44, 56–80. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.T.; Iwaniec, U.T.; Andrade, J.E.; Branscum, A.J.; Neese, S.L.; Olson, D.A.; Wagner, L.; Wang, V.C.; Schantz, S.L.; Helferich, W.G. Genistein administered as a once-daily oral supplement had no beneficial effect on the tibia in rat models for postmenopausal bone loss. Menopause 2013, 20, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Carneiro-Filho, B.A.; Lima, I.P.; Araujo, D.H.; Cavalcante, M.C.; Carvalho, G.H.; Brito, G.A.; Lima, V.; Monteiro, S.M.; Santos, F.N.; Ribeiro, R.A.; et al. Intestinal barrier function and secretion in methotrexate-induced rat intestinal mucositis. Dig. Dis. Sci. 2004, 49, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Xian, C.J. Roles of growth factors in chemotherapy-induced intestinal mucosal damage repair. Curr. Pharm. Biotechnol. 2003, 4, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Burnett, B.P.; Polito, F.; Levy, R.M.; Marini, H.; di Stefano, V.; Irrera, N.; Armbruster, M.A.; Minutoli, L.; Altavilla, D.; et al. Genistein aglycone reverses glucocorticoid-induced osteoporosis and increases bone breaking strength in rats: A comparative study with alendronate. Br. J. Pharmacol. 2009, 156, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Fanti, P.; Monier-Faugere, M.C.; Geng, Z.; Schmidt, J.; Morris, P.E.; Cohen, D.; Malluche, H.H. The phytoestrogen genistein reduces bone loss in short-term ovariectomized rats. Osteoporos. Int. 1998, 8, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Villani, F.; Viola, G.; Vismara, C.; Laffranchi, A.; di Russo, A.; Viviani, S.; Bonfante, V. Lung function and serum concentrations of different cytokines in patients submitted to radiotherapy and intermediate/high dose chemotherapy for hodgkin's disease. Anticancer Res. 2002, 22, 2403–2408. [Google Scholar] [PubMed]
- Kawagishi, C.; Kurosaka, K.; Watanabe, N.; Kobayashi, Y. Cytokine production by macrophages in association with phagocytosis of etoposide-treated p388 cells in vitro and in vivo. Biochim. Biophys. Acta 2001, 1541, 221–230. [Google Scholar] [CrossRef]
- Karieb, S.; Fox, S.W. Phytoestrogens directly inhibit TNF-α-induced bone resorption in RAW264.7 cells by suppressing c-fos-induced NFATc1 expression. J. Cell. Biochem. 2011, 112, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Arao, Y.; Sun, S.J.; Kikuchi, A.; Kayama, F. Oral administration of soy-derived genistin suppresses lipopolysaccharide-induced acute liver inflammation but does not induce thymic atrophy in the rat. Life Sci. 2006, 78, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Droke, E.A.; Hager, K.A.; Lerner, M.R.; Lightfoot, S.A.; Stoecker, B.J.; Brackett, D.J.; Smith, B.J. Soy isoflavones avert chronic inflammation-induced bone loss and vascular disease. J. Inflamm. (Lond.) 2007, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, E.; Yamaguchi, M. Anabolic effect of genistein in osteoblastic MC3T3-E1 cells. Int. J. Mol. Med. 2000, 5, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Jin, T.Y.; Jin, W.F.; Gu, S.Z.; Zhou, Y.F. Modulation of isoflavones on bone-nodule formation in rat calvaria osteoblasts in vitro. Biomed. Environ. Sci. 2003, 16, 83–89. [Google Scholar] [PubMed]
- Li, Y.Q.; Xing, X.H.; Wang, H.; Weng, X.L.; Yu, S.B.; Dong, G.Y. Dose-dependent effects of genistein on bone homeostasis in rats' mandibular subchondral bone. Acta Pharmacol. Sin. 2012, 33, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.C.; Xiao, Z.S.; Qin, Y.F.; Zhou, H.H. Genistein stimulates osteoblastic differentiation via p38 MAPK-Cbfa1 pathway in bone marrow culture. Acta Pharmacol. Sin. 2007, 28, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Naaz, A.; Yellayi, S.; Zakroczymski, M.A.; Bunick, D.; Doerge, D.R.; Lubahn, D.B.; Helferich, W.G.; Cooke, P.S. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology 2003, 144, 3315–3320. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Ma, Y.; Sheng, Z.; Jin, Y.; Zhang, Y.; Fang, L.; Fan, H.; Liao, E. Effects of genistein on vertebral trabecular bone microstructure, bone mineral density, microcracks, osteocyte density, and bone strength in ovariectomized rats. J. Bone Miner. Metab. 2008, 26, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Sehmisch, S.; Erren, M.; Kolios, L.; Tezval, M.; Seidlova-Wuttke, D.; Wuttke, W.; Stuermer, K.M.; Stuermer, E.K. Effects of isoflavones equol and genistein on bone quality in a rat osteopenia model. Phytother. Res. 2010, 24 (Suppl. 2), S168–S174. [Google Scholar] [CrossRef] [PubMed]
- Shike, M.; Doane, A.S.; Russo, L.; Cabal, R.; Reis-Filho, J.S.; Gerald, W.; Cody, H.; Khanin, R.; Bromberg, J.; Norton, L. The effects of soy supplementation on gene expression in breast cancer: A randomized placebo-controlled study. J. Natl. Cancer Inst. 2014, 106, dju189. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Brown, N.M.; Zhao, X.; Lindley, S.L.; Heubi, J.E.; King, E.C.; Messina, M.J. Soy isoflavone phase II metabolism differs between rodents and humans: Implications for the effect on breast cancer risk. Am. J. Clin. Nutr. 2011, 94, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.M.; Shandala, T.; Nguyen, L.; Muhlhausler, B.S.; Chen, K.M.; Howe, P.R.; Xian, C.J. Effects of resveratrol supplementation on bone growth in young rats and microarchitecture and remodeling in ageing rats. Nutrients 2014, 6, 5871–5887. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, T.J.; Shandala, T.; Lee, A.M.; Foster, B.K.; Chen, K.-M.; Howe, P.R.; Xian, C.J. Potential Effects of Phytoestrogen Genistein in Modulating Acute Methotrexate Chemotherapy-Induced Osteoclastogenesis and Bone Damage in Rats. Int. J. Mol. Sci. 2015, 16, 18293-18311. https://doi.org/10.3390/ijms160818293
King TJ, Shandala T, Lee AM, Foster BK, Chen K-M, Howe PR, Xian CJ. Potential Effects of Phytoestrogen Genistein in Modulating Acute Methotrexate Chemotherapy-Induced Osteoclastogenesis and Bone Damage in Rats. International Journal of Molecular Sciences. 2015; 16(8):18293-18311. https://doi.org/10.3390/ijms160818293
Chicago/Turabian StyleKing, Tristan J., Tetyana Shandala, Alice M. Lee, Bruce K. Foster, Ke-Ming Chen, Peter R. Howe, and Cory J. Xian. 2015. "Potential Effects of Phytoestrogen Genistein in Modulating Acute Methotrexate Chemotherapy-Induced Osteoclastogenesis and Bone Damage in Rats" International Journal of Molecular Sciences 16, no. 8: 18293-18311. https://doi.org/10.3390/ijms160818293
APA StyleKing, T. J., Shandala, T., Lee, A. M., Foster, B. K., Chen, K. -M., Howe, P. R., & Xian, C. J. (2015). Potential Effects of Phytoestrogen Genistein in Modulating Acute Methotrexate Chemotherapy-Induced Osteoclastogenesis and Bone Damage in Rats. International Journal of Molecular Sciences, 16(8), 18293-18311. https://doi.org/10.3390/ijms160818293