Tangential Flow Ultrafiltration Allows Purification and Concentration of Lauric Acid-/Albumin-Coated Particles for Improved Magnetic Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Filtration and Concentration Efficiency




2.2. Effect of Filtration on Magnetic Properties of SEONLA-BSA

2.3. Effect of Filtration on Colloidal Stability and Cellular Uptake


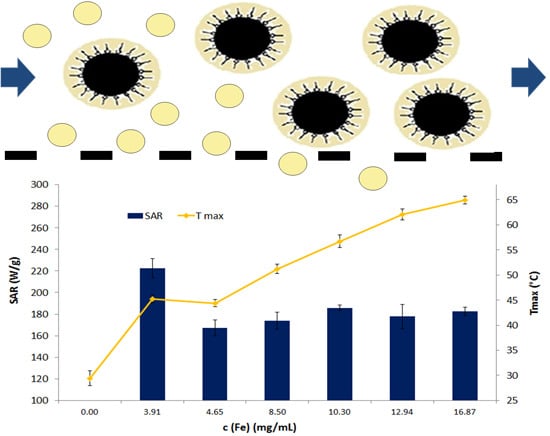
2.4. Dependence of SAR and Tmax on Filtration and Iron Oxide Concentration

3. Experimental Section
3.1. Materials and Chemicals
3.2. Nanoparticles and Filtration
3.3. PCCS and Zeta Potential Measurements
3.4. Vibrating Sample Magnetometry
3.5. Blood Stability Assays
3.6. Cellular Uptake Assays
3.7. SAR and Tmax Determination
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lyer, S.; Tietze, R.; Jurgons, R.; Struffert, T.; Engelhorn, T.; Schreiber, E.; Dorfler, A.; Alexiou, C. Visualisation of tumour regression after local chemotherapy with magnetic nanoparticles—A pilot study. Anticancer Res. 2010, 30, 1553–1557. [Google Scholar] [PubMed]
- Lee, I.J.; Ahn, C.H.; Cha, E.J.; Chung, I.J.; Chung, J.W.; Kim, Y.I. Improved drug targeting to liver tumors after intra-arterial delivery using superparamagnetic iron oxide and iodized oil: Preclinical study in a rabbit model. Investig. Radiol. 2013, 48, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Dutz, S.; Hafeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Durr, S.; Schmidt, W.; Janko, C.; Kraemer, H.P.; Tripal, P.; Eiermann, F.; Tietze, R.; Lyer, S.; Alexiou, C. A novel magnetic field device for inducing hyperthermia using magnetic nanoparticles. Biomed. Tech. 2013, 58. [Google Scholar] [CrossRef] [PubMed]
- Stapf, M.; Pömpner, N.; Kettering, M.; Hilger, I. Magnetic thermoablation stimuli alter BCL2 and FGF-R1 but not HSP70 expression profiles in BT474 breast tumors. Int. J. Nanomed. 2015, 10, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Laurent, S.; Fattahi, H.; Vander Elst, L.; Muller, R.N. Superparamagnetic nanosystems based on iron oxide nanoparticles for biomedical imaging. Nanomedicine (London, England) 2011, 6, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.; Wiekhorst, F.; Schwarz, K.; Lyer, S.; Tietze, R.; Alexiou, C.; Trahms, L. Magnetorelaxometric quantification of magnetic nanoparticles in an artery model after ex vivo magnetic drug targeting. Phys. Med. Biol. 2009, 54, N417–N424. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.Y.; Illes, E.; Bauer, R.A.; Nesztor, D.; Szekeres, M.; Zupko, I.; Tombacz, E. Designed polyelectrolyte shell on magnetite nanocore for dilution-resistant biocompatible magnetic fluids. Langmuir 2012, 28, 16638–16646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Wolf, D.; Odenbach, S. A rheological and microscopical characterization of biocompatible ferrofluids. J. Magn. Magn. Mater. 2014, 354, 98–104. [Google Scholar] [CrossRef]
- Zaloga, J.; Janko, C.; Nowak, J.; Matuszak, J.; Knaup, S.; Eberbeck, D.; Tietze, R.; Unterweger, H.; Friedrich, R.P.; Duerr, S.; et al. Development of a lauric acid/albumin hybrid iron oxide nanoparticle system with improved biocompatibility. Int. J. Nanomed. 2014, 9, 4847–4866. [Google Scholar] [CrossRef] [PubMed]
- Faulds, D.; Balfour, J.; Chrisp, P.; Langtry, H. Mitoxantrone. Drugs 1991, 41, 400–449. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Wiekhorst, F.; Trahms, L.; Odenbach, S. The influence of hydrodynamic diameter and core composition on the magnetoviscous effect of biocompatible ferrofluids. J. Phys. Condens. Matter 2014, 26. [Google Scholar] [CrossRef] [PubMed]
- Gitter, K.; Odenbach, S. Simulation of magnetic drug targeting for a branched artery-model with non-newtonian flow behaviour of blood. Magnetohydrodynamics 2013, 49, 541–545. [Google Scholar]
- David, A.E.; Cole, A.J.; Chertok, B.; Park, Y.S.; Yang, V.C. A combined theoretical and in vitro modeling approach for predicting the magnetic capture and retention of magnetic nanoparticles in vivo. J. Control. Release 2011, 152, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Dalwadi, G.; Benson, H.A.; Chen, Y. Comparison of diafiltration and tangential flow filtration for purification of nanoparticle suspensions. Pharm. Res. 2005, 22, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.B.; Baker, J.D.; Stahler, A.C.; Williams, A.J.; Sisco, J.N.; Trefry, J.C.; Wooley, D.P.; Pavel Sizemore, I.E. Tangential flow ultrafiltration: A “green” method for the size selection and concentration of colloidal silver nanoparticles. J. Vis. Exp. 2012, 68. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Toth, I.Y.; Illes, E.; Hajdu, A.; Zupko, I.; Farkas, K.; Oszlanczi, G.; Tiszlavicz, L.; Tombacz, E. Chemical and colloidal stability of carboxylated core-shell magnetite nanoparticles designed for biomedical applications. Int. J. Mol. Sci. 2013, 14, 14550–14574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosicka, D.; Sembera, J. Changes in the nanoparticle aggregation rate due to the additional effect of electrostatic and magnetic forces on mass transport coefficients. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Textor, M.; Reimhult, E. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 2011, 3, 2819–2843. [Google Scholar] [CrossRef] [PubMed]
- Tombácz, E.; Tóth, I.Y.; Nesztor, D.; Illés, E.; Hajdú, A.; Szekeres, M.; Vékás, L. Adsorption of organic acids on magnetite nanoparticles, pH-dependent colloidal stability and salt tolerance. Colloids Surf. A Physicochem. Eng. Asp. 2013, 435, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Gebauer, J.S.; Malissek, M.; Simon, S.; Knauer, S.K.; Maskos, M.; Stauber, R.H.; Peukert, W.; Treuel, L. Impact of the nanoparticle-protein corona on colloidal stability and protein structure. Langmuir 2012, 28, 9673–9679. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, A.; Fenaroli, F.; Monopoli, M.P.; Åberg, C.; Dawson, K.A.; Salvati, A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 2012, 6, 5845–5857. [Google Scholar] [CrossRef] [PubMed]
- Dadashzadeh, E.R.; Hobson, M.; Henry Bryant, L., Jr.; Dean, D.D.; Frank, J.A. Rapid spectrophotometric technique for quantifying iron in cells labeled with superparamagnetic iron oxide nanoparticles: Potential translation to the clinic. Contrast Media Mol. Imaging 2013, 8, 50–56. [Google Scholar] [PubMed]
- Ludwig, R.; Stapf, M.; Dutz, S.; Muller, R.; Teichgraber, U.; Hilger, I. Structural properties of magnetic nanoparticles determine their heating behavior—An estimation of the in vivo heating potential. Nanoscale Res. Lett. 2014, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, G.; Mella, O.; Roszinski, S.; Weiss, C.; Wagner, T. Hyperthermia enhances mitoxantrone cytotoxicity on human breast carcinoma and sarcoma xenografts in nude mice. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 669–673. [Google Scholar] [CrossRef]
- Tietze, R.; Lyer, S.; Durr, S.; Struffert, T.; Engelhorn, T.; Schwarz, M.; Eckert, E.; Goen, T.; Vasylyev, S.; Peukert, W.; Wiekhorst, F.; Trahms, L.; Dorfler, A.; Alexiou, C. Efficient drug-delivery using magnetic nanoparticles—Biodistribution and therapeutic effects in tumour bearing rabbits. Nanomedicine 2013, 9, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, C.; Jurgons, R.; Schmid, R.J.; Bergemann, C.; Henke, J.; Erhard, W.; Huenges, E.; Parak, F. Magnetic Drug Targeting—Biodistribution of the magnetic carrier and the chemotherapeutic agent mitoxantrone after locoregional cancer treatment. J. Drug Target. 2003, 11, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Salgin, S.; Salgın, U.; Bahadir, S. Zeta Potentials and Isoelectric Points of Biomolecules: The Effects of Ion Types and Ionic Strengths. Int. J. Electrochem. Sci. 2012, 7, 12404–12414. [Google Scholar]
- Spectrumlabs Pore Size Chart. Available online: http://eu.spectrumlabs.com/filtration/PoreSize.html (accessed on 28 July 2015).
- Kolorimetrie-Photometrie. Available online: http://www.lickl.net/doku/photo.pdf (accessed on 8 June 2014).
- Friedrich, R.P.; Janko, C.; Poettler, M.; Tripal, P.; Zaloga, J.; Cicha, I.; Durr, S.; Nowak, J.; Odenbach, S.; Slabu, I.; et al. Flow cytometry for intracellular SPION quantification: Specificity and sensitivity in comparison with spectroscopic methods. Int. J. Nanomed. 2015, 10, 4185–4201. [Google Scholar] [CrossRef] [PubMed]
- Teran, F.J.; Casado, C.; Mikuszeit, N.; Salas, G.; Bollero, A.; Morales, M.P.; Camarero, J.; Miranda, R. Accurate determination of the specific absorption rate in superparamagnetic nanoparticles under non-adiabatic conditions. Appl. Phys. Lett. 2012, 101. [Google Scholar] [CrossRef] [Green Version]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaloga, J.; Stapf, M.; Nowak, J.; Pöttler, M.; Friedrich, R.P.; Tietze, R.; Lyer, S.; Lee, G.; Odenbach, S.; Hilger, I.; et al. Tangential Flow Ultrafiltration Allows Purification and Concentration of Lauric Acid-/Albumin-Coated Particles for Improved Magnetic Treatment. Int. J. Mol. Sci. 2015, 16, 19291-19307. https://doi.org/10.3390/ijms160819291
Zaloga J, Stapf M, Nowak J, Pöttler M, Friedrich RP, Tietze R, Lyer S, Lee G, Odenbach S, Hilger I, et al. Tangential Flow Ultrafiltration Allows Purification and Concentration of Lauric Acid-/Albumin-Coated Particles for Improved Magnetic Treatment. International Journal of Molecular Sciences. 2015; 16(8):19291-19307. https://doi.org/10.3390/ijms160819291
Chicago/Turabian StyleZaloga, Jan, Marcus Stapf, Johannes Nowak, Marina Pöttler, Ralf P. Friedrich, Rainer Tietze, Stefan Lyer, Geoffrey Lee, Stefan Odenbach, Ingrid Hilger, and et al. 2015. "Tangential Flow Ultrafiltration Allows Purification and Concentration of Lauric Acid-/Albumin-Coated Particles for Improved Magnetic Treatment" International Journal of Molecular Sciences 16, no. 8: 19291-19307. https://doi.org/10.3390/ijms160819291
APA StyleZaloga, J., Stapf, M., Nowak, J., Pöttler, M., Friedrich, R. P., Tietze, R., Lyer, S., Lee, G., Odenbach, S., Hilger, I., & Alexiou, C. (2015). Tangential Flow Ultrafiltration Allows Purification and Concentration of Lauric Acid-/Albumin-Coated Particles for Improved Magnetic Treatment. International Journal of Molecular Sciences, 16(8), 19291-19307. https://doi.org/10.3390/ijms160819291