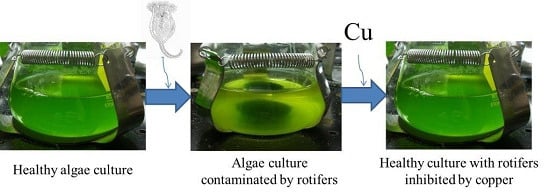
Use of Copper to Selectively Inhibit Brachionus calyciflorus (Predator) Growth in Chlorella kessleri (Prey) Mass Cultures for Algae Biodiesel Production
Abstract
:1. Introduction

2. Results and Discussion
2.1. Effect of Copper on B. calyciflorus and C. kessleri

2.2. C. kessleri Pond Crash Rate



2.3. C. kessleri—B. calyciflorus Co-Culture Copper Toxicity Test


4. Experimental Section
4.1. Algae and Rotifer Preparation
4.2. Copper Toxicity and B. calyciflorus
4.3. Copper Toxicity and C. kessleri
4.4. C. kessleri Pond Crash Rate Tests Using B. calyciflorus
4.5. C. kessleri and B. calyciflorus Co-Culture Copper Toxicity Tests
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jones, C.S.; Mayfield, S.P. Algae biofuels: Versatility for the future of bioenergy. Curr. Opin. Biotechnol. 2012, 23, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Lubzens, E. Raising rotifers for use in aquaculture. Hydrobiologia 1987, 147, 245–255. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Aoki, S.; Maeda, M.; Hino, A. Competition between the rotifer Brachionus rotundiformis and the ciliate Euplotes vannus fed on two different algae. Aquaculture 2004, 241, 331–343. [Google Scholar] [CrossRef]
- Snell, T.W.; Janssen, C.R. Rotifers in ecotoxicology: A review. Hydrobiologia 1995, 313, 231–247. [Google Scholar] [CrossRef]
- Snell, T.W.; Persoone, G. Acute toxicity bioassays using rotifers. I. A test for brackish and marine environments with Brachionus plicatilis. Aquat. Toxicol. 1989, 14, 65–80. [Google Scholar] [CrossRef]
- Snell, T.W.; Persoone, G. Acute toxicity bioassays using rotifers. II. A freshwater test with Brachionus rubens. Aquat. Toxicol. 1989, 14, 81–91. [Google Scholar] [CrossRef]
- Snell, T.W.; Moffat, B.D.; Janssen, C.; Persoone, G. Acute toxicity tests using rotifers: IV. Effects of cyst age, temperature, and salinity on the sensitivity of Brachionus calyciflorus. Ecotoxicol. Environ. Saf. 1991, 21, 308–317. [Google Scholar] [CrossRef]
- Franklin, N.M.; Stauber, J.L.; Markich, S.J.; Lim, R.P. pH-dependent toxicity of copper and uranium to a tropical freshwater alga (Chlorella sp.). Aquat. Toxicol. 2000, 48, 275–289. [Google Scholar] [CrossRef]
- Franklin, N.M.; Stauber, J.L.; Markich, S.J.; Lim, R. A new tropical algal test to assess the toxicity of metals in freshwaters. In Supervising Scientist Report; 1998; 133. Available online http://www.environment.gov.au/science/supervising-scientist/publications/ssr/new-tropical-algal-test-assess-toxicity-metals-freshwaters (accessed on 30 June 2015). [Google Scholar]
- Ansari, Z.; Amjad, M. Copper toxicity to algae (Chlorella vulgaris) growth. Pakistan J. Sci. 2008, 60, 64–66. [Google Scholar]
- Bishnoi, N.R.; Garima, A.P. Biosorption of copper from aqueous solution using algal biomass. J. Sci. Indust. Res. 2004, 63, 813–816. [Google Scholar]
- Qian, H.; Yu, S.; Sun, Z.; Xie, X.; Liu, W.; Fu, Z. Effects of copper sulfate, hydrogen peroxide and N-phenyl-2-naphthylamine on oxidative stress and the expression of genes involved photosynthesis and microcystin disposition in Microcystis aeruginosa. Aquat. Toxicol. 2010, 99, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.D.; Creed, J.T.; Brockhoff, C.A. Sample preparation procedure for spectrochemical determination of total recoverable elements, Revision 2.8, EMMC Version. 1994. Available online: http://water.epa.gov/scitech/methods/cwa/bioindicators/upload/2007_07_10_methods_method_200_2.pdf (accessed on 30 June 2015).
- Van Wychen, S.; Laurens, L.M.L. Determination of total lipids as fatty acid methyl esters (FAME) by in situ transesterification. Contract 2013, 303, 375–300. [Google Scholar]
- Kantz, T.; Bold, H. Morphological and Taxonomic Investigations of Nostoc and Anabaena in Culture, Phycological Studies IX; University of Texas Publishers: Austin, Texas, USA, 1969; pp. 1–67. [Google Scholar]
- Zhu, C.J.; Lee, Y.K. Determination of biomass dry weight of marine microalgae. J. Appl. Phycol. 1997, 9, 189–194. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradeep, V.; Van Ginkel, S.W.; Park, S.; Igou, T.; Yi, C.; Fu, H.; Johnston, R.; Snell, T.; Chen, Y. Use of Copper to Selectively Inhibit Brachionus calyciflorus (Predator) Growth in Chlorella kessleri (Prey) Mass Cultures for Algae Biodiesel Production. Int. J. Mol. Sci. 2015, 16, 20674-20684. https://doi.org/10.3390/ijms160920674
Pradeep V, Van Ginkel SW, Park S, Igou T, Yi C, Fu H, Johnston R, Snell T, Chen Y. Use of Copper to Selectively Inhibit Brachionus calyciflorus (Predator) Growth in Chlorella kessleri (Prey) Mass Cultures for Algae Biodiesel Production. International Journal of Molecular Sciences. 2015; 16(9):20674-20684. https://doi.org/10.3390/ijms160920674
Chicago/Turabian StylePradeep, Vishnupriya, Steven W. Van Ginkel, Sichoon Park, Thomas Igou, Christine Yi, Hao Fu, Rachel Johnston, Terry Snell, and Yongsheng Chen. 2015. "Use of Copper to Selectively Inhibit Brachionus calyciflorus (Predator) Growth in Chlorella kessleri (Prey) Mass Cultures for Algae Biodiesel Production" International Journal of Molecular Sciences 16, no. 9: 20674-20684. https://doi.org/10.3390/ijms160920674
APA StylePradeep, V., Van Ginkel, S. W., Park, S., Igou, T., Yi, C., Fu, H., Johnston, R., Snell, T., & Chen, Y. (2015). Use of Copper to Selectively Inhibit Brachionus calyciflorus (Predator) Growth in Chlorella kessleri (Prey) Mass Cultures for Algae Biodiesel Production. International Journal of Molecular Sciences, 16(9), 20674-20684. https://doi.org/10.3390/ijms160920674