The Effect of Coatings on the Affinity of Lanthanide Nanoparticles to MKN45 and HeLa Cancer Cells and Improvement in Photodynamic Therapy Efficiency
Abstract
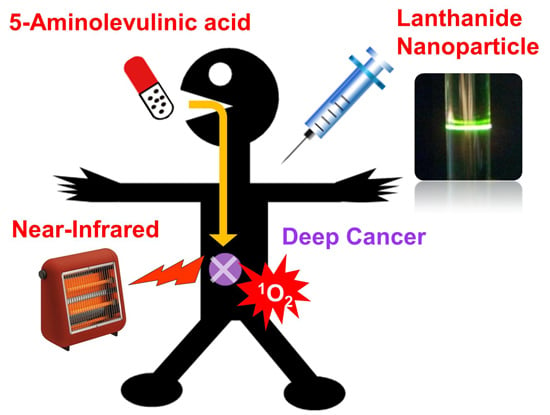
:1. Introduction


2. Results and Discussion
2.1. Characterization of LNPs


2.2. Affinity of the Surface Modified LNPs with a Cancer Cell

2.3. PDT Effects of Amino-Modified LNPs toward MKN45

3. Experimental Section
3.1. General
3.2. Adhesibility of LNP to Cells
3.3. In Vitro PDT Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.-I.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic acid (ALA) as a prodrug in photodynamic therapy of cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef]
- Harris, F.; Pierpoint, L. Photodynamic therapy based on 5-aminolevulinic acid and its use as an antimicrobial agent. Med. Res. Rev. 2012, 32, 1292–1327. [Google Scholar] [CrossRef] [PubMed]
- Tetard, M.-C.; Vermandel, M.; Mordon, S.; Lejeune, J.-P.; Reyns, N. Experimental use of photodynamic therapy in high grade gliomas: A review focused on 5-aminolevulinic acid. Photodiagn. Photodyn. Ther. 2014, 11, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, T.; Ogilby, P.R.; Lambert, J.D.C. Effect of intracellular photosensitized singlet oxygen production on the electrophysiological properties of cultured rat hippocampal neurons. Photochem. Photobiol. Sci. 2010, 9, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Ritz, J.-P.; Roggan, A.; Isbert, C.; Müller, G.; Buhr, H.J.; Germer, C.-T. Optical properties of native and coagulated porcine liver tissue between 400 and 2400 nm. Lasers Surg. Med. 2001, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Lozovaya, G.I.; Masinovsky, Z.; Sivash, A.A. Protoporphyrin IX as a possible ancient photosensitizer: Spectral and photochemical studies. Orig. Life Evol. Biosph. 1990, 20, 321–330. [Google Scholar] [CrossRef]
- Shimoyama, A.; Watase, H.; Liu, Y.; Ogura, S.-I.; Hagiya, Y.; Takahashi, K.; Inoue, K.; Tanaka, T.; Murayama, Y.; Otsuji, E.; et al. Access to a novel near-infrared photodynamic therapy through the combined use of 5-aminolevulinic acid and lanthanide nanoparticles. Photodiagn. Photodyn. Ther. 2013, 10, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F. Photon Upconversion Nanomaterials; Springer: Heidelberg, Germany, 2015; pp. 73–119. [Google Scholar]
- Liu, X.; Yan, C.-H.; Capobianco, J.A. Photon upconversion nanomaterials (the editorial for a special issue on the title subject). Chem. Soc. Rev. 2015, 44, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kobayashi, T.; Iizuka, M.; Tanaka, T.; Sotokawa, I.; Shimoyama, A.; Murayama, Y.; Otsuji, E.; Ohkubo, A.; Ogura, S.-I.; et al. Sugar-attached upconversion lanthanide nanoparticles: A novel tool for high-throughput lectin assay. Bioorg. Med. Chem. 2013, 21, 2832–2842. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.; Wang, J.; Xu, J.; Chen, H.; Zhang, C.; Hong, M.; Liu, X. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Auzel, F. Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 2004, 104, 139–173. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yu, J.; Ma, E.; Lin, K. Synthesis and characterization of highly efficient near-infrared upconversion Sc3+/Er3+/Yb3+ tridoped NaYF4. J. Phys. Chem. C 2010, 114, 4719–4724. [Google Scholar] [CrossRef]
- Jalil, R.A.; Zhang, Y. Biocompatibility of silica coated NaYF4 upconversion fluorescent nanocrystals. Biomaterials 2008, 29, 4122–4128. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Mi, C.-C.; Wang, W.-X.; Liu, C.-H.; Wu, Y.-F.; Xu, Z.-R.; Mao, C.-B.; Xu, S.-K. Immunolabeling and NIR-excited fluorescent imaging of HeLa cells by using NaYF4:Yb, Er upconversion nanoparticles. ACS Nano 2009, 3, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Hartgrink, H.H.; Jansen, E.P.M.; van Grieken, N.C.T.; van de Velde, C.J.H. Gastric cancer. Lancet 2009, 374, 477–490. [Google Scholar] [CrossRef]
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharmacol. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Dausend, J.; Musyanovych, A.; Dass, M.; Walther, P.; Schrezenmeier, H.; Landfester, K.; Mailänder, V. Uptake mechanisum of oppositely charged fluorescent nanoparticles in HeLa cells. Macromol. Biosci. 2008, 8, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, L.; Li, C.; Li, F. A versatile fabrication of upconversion nanophosphors with functional-surface tunable ligands. J. Mater. Chem. 2010, 20, 8078–8085. [Google Scholar] [CrossRef]
- Allfrey, V.; Teply, L.J.; Geffen, C.; King, C.G. A fluorometric method for the determination of pteroylglutamic acid. J. Biol. Chem. 1949, 178, 465–481. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawamura, T.; Tanaka, T.; Ishige, H.; Iizuka, M.; Murayama, Y.; Otsuji, E.; Ohkubo, A.; Ogura, S.-I.; Yuasa, H. The Effect of Coatings on the Affinity of Lanthanide Nanoparticles to MKN45 and HeLa Cancer Cells and Improvement in Photodynamic Therapy Efficiency. Int. J. Mol. Sci. 2015, 16, 22415-22424. https://doi.org/10.3390/ijms160922415
Sawamura T, Tanaka T, Ishige H, Iizuka M, Murayama Y, Otsuji E, Ohkubo A, Ogura S-I, Yuasa H. The Effect of Coatings on the Affinity of Lanthanide Nanoparticles to MKN45 and HeLa Cancer Cells and Improvement in Photodynamic Therapy Efficiency. International Journal of Molecular Sciences. 2015; 16(9):22415-22424. https://doi.org/10.3390/ijms160922415
Chicago/Turabian StyleSawamura, Takashi, Tatsumi Tanaka, Hiroyuki Ishige, Masayuki Iizuka, Yasutoshi Murayama, Eigo Otsuji, Akihiro Ohkubo, Shun-Ichiro Ogura, and Hideya Yuasa. 2015. "The Effect of Coatings on the Affinity of Lanthanide Nanoparticles to MKN45 and HeLa Cancer Cells and Improvement in Photodynamic Therapy Efficiency" International Journal of Molecular Sciences 16, no. 9: 22415-22424. https://doi.org/10.3390/ijms160922415
APA StyleSawamura, T., Tanaka, T., Ishige, H., Iizuka, M., Murayama, Y., Otsuji, E., Ohkubo, A., Ogura, S. -I., & Yuasa, H. (2015). The Effect of Coatings on the Affinity of Lanthanide Nanoparticles to MKN45 and HeLa Cancer Cells and Improvement in Photodynamic Therapy Efficiency. International Journal of Molecular Sciences, 16(9), 22415-22424. https://doi.org/10.3390/ijms160922415