Fluence Rate Differences in Photodynamic Therapy Efficacy and Activation of Epidermal Growth Factor Receptor after Treatment of the Tumor-Involved Murine Thoracic Cavity
Abstract
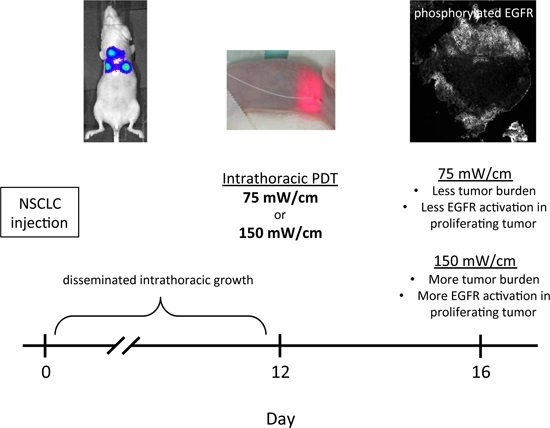
:1. Introduction
2. Results and Discussion
2.1. Fluence Rate Effects on Efficacy of Thoracic Photodynamic Therapy (PDT)


2.2. Imaging to Identify Nodules with Incomplete PDT Response

2.3. PDT-Induced Epidermal Growth Factor Receptor (EGFR) Signaling

2.4. EGFR Activation in Proliferating Tissue


2.5. Discussion
3. Experimental Section
3.1. Cell Line and Tumor Propagation
3.2. Photodynamic Therapy
3.3. Optical Imaging
3.4. Immunohistochemistry
3.5. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Quirk, B.J.; Brandal, G.; Donlon, S.; Vera, J.C.; Mang, T.S.; Foy, A.B.; Lew, S.M.; Girotti, A.W.; Jogal, S.; la Violette, P.S.; et al. Photodynamic therapy (PDT) for malignant brain tumors—Where do we stand? Photodiagn. Photodyn. Ther. 2015, 12, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.M.; Fraker, D.L.; Mick, R.; Metz, J.; Busch, T.M.; Smith, D.; Zhu, T.; Rodriguez, C.; Dimofte, A.; Spitz, F.; et al. A phase II trial of intraperitoneal photodynamic therapy for patients with peritoneal carcinomatosis and sarcomatosis. Clin. Cancer Res. 2006, 12, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, J.S.; Culligan, M.J.; Mick, R.; Stevenson, J.; Hahn, S.M.; Sterman, D.; Punekar, S.; Glatstein, E.; Cengel, K. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann. Thorac. Surg. 2012, 93, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, J.S.; Mick, R.; Stevenson, J.P.; Zhu, T.; Busch, T.M.; Shin, D.; Smith, D.; Culligan, M.; Dimofte, A.; Glatstein, E.; et al. Phase II trial of pleural photodynamic therapy and surgery for patients with non-small-cell lung cancer with pleural spread. J. Clin. Oncol. 2004, 22, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Patterson, M.S. The physics, biophysics and technology of photodynamic therapy. Phys. Med. Biol. 2008, 53, R61–R109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.C.; Liang, X.; Sandell, J.; Finlay, J.C.; Dimofte, A.; Rodriguez, C.; Cengel, K.; Friedberg, J.; Hahn, S.M.; Glatstein, E. A real-time treatment guidance system for pleural PDT. Proc. SPIE Int. Soc. Opt. Eng. 2012, 8210. [Google Scholar] [CrossRef]
- Dimofte, A.; Zhu, T.C.; Finlay, J.C.; Cullighan, M.; Edmonds, C.E.; Friedberg, J.S.; Cengel, K.; Hahn, S.M. In vivo light dosimetry for HPPH-mediated pleural PDT. Proc. SPIE Int. Soc. Opt. Eng. 2010, 7551. [Google Scholar] [CrossRef]
- Coutier, S.; Bezdetnaya, L.N.; Foster, T.H.; Parache, R.M.; Guillemin, F. Effect of irradiation fluence rate on the efficacy of photodynamic therapy and tumor oxygenation in meta-tetra (hydroxyphenyl) chlorin (mTHPC)-sensitized HT29 xenografts in nude mice. Radiat. Res. 2002, 158, 339–345. [Google Scholar] [CrossRef]
- Busch, T.M.; Xing, X.; Yu, G.; Yodh, A.; Wileyto, E.P.; Wang, H.W.; Durduran, T.; Zhu, T.C.; Wang, K.K. Fluence rate-dependent intratumor heterogeneity in physiologic and cytotoxic responses to Photofrin photodynamic therapy. Photochem. Photobiol. Sci. 2009, 8, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Kruijt, B.; van der Ploeg-van den Heuvel, A.; de Bruijn, H.S.; Sterenborg, H.J.; Amelink, A.; Robinson, D.J. Monitoring interstitial m-THPC-PDT in vivo using fluorescence and reflectance spectroscopy. Lasers Surg. Med. 2009, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Gollnick, S.O.; Snyder, J.W.; Busch, T.M.; Kousis, P.C.; Cheney, R.T.; Morgan, J. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004, 64, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; di Venosa, G.; Hasan, T.; Al, B. Mechanisms of resistance to photodynamic therapy. Curr. Med. Chem. 2011, 18, 2486–2515. [Google Scholar] [CrossRef] [PubMed]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.M.; Hamblin, M.R.; Heger, M. Tumor cell survival pathways activated by photodynamic therapy: A molecular basis for pharmacological inhibition strategies. Cancer Metastasis Rev. 2015, 34, 643–690. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Carpio, P.A.; Trelles, M.A. The role of epidermal growth factor receptor in photodynamic therapy: A review of the literature and proposal for future investigation. Lasers Med. Sci. 2010, 25, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Banik, N.L.; Ray, S.K. Photofrin based photodynamic therapy and miR-99a transfection inhibited FGFR3 and PI3K/Akt signaling mechanisms to control growth of human glioblastoma in vitro and in vivo. PLoS ONE 2013, 8, e55652. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, R.; Girotti, A.W. Cytoprotective signaling associated with nitric oxide upregulation in tumor cells subjected to photodynamic therapy-like oxidative stress. Free Radic. Biol. Med. 2013, 57, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; Gomer, C.J. Targeting the 90 kDa heat shock protein improves photodyamic therapy. Cancer Lett. 2010, 289, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Weyergang, A.; Selbo, P.K.; Berg, K. Sustained ERK inhibition by EGFR targeting therapies is a predictive factor for synergistic cytotoxicity with PDT as neoadjuvant therapy. Biochim. Biophys. Acta 2013, 1830, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, R.; Gan, Y.Y.; Soo, K.C.; Olivo, M. The effect of photodynamic therapy on tumor angiogenesis. Cell. Mol. Life Sci. 2009, 66, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Gallagher-Colombo, S.M.; Miller, J.; Cengel, K.A.; Putt, M.E.; Vinogradov, S.A.; Busch, T.M. Erlotinib pretreatment improves photodynamic therapy of non-small cell lung carcinoma xenografts via multiple mechanisms. Cancer Res. 2015, 75, 3118–3126. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, R.; Ng, Q.F.; Thong, P.S.; Soo, K.C. Nimotuzumab increases the anti-tumor effect of photodynamic therapy in an oral tumor model. Oncotarget 2015, 6, 13487–13505. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen, M.G.; Rizvi, I.; Chang, Y.; Moor, A.C.; Oliva, E.; Sherwood, M.; Pogue, B.; Hasan, T. Synergism of epidermal growth factor receptor-targeted immunotherapy with photodynamic treatment of ovarian cancer in vivo. J. Natl. Cancer Inst. 2005, 97, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Gallagher-Colombo, S.M.; Maas, A.L.; Yuan, M.; Busch, T.M. Photodynamic therapy-induced angiogenic signaling: Consequences and solutions to improve therapeutic response. Isr. J. Chem. 2012, 52, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Grossman, C.E.; Pickup, S.; Durham, A.; Wileyto, E.P.; Putt, M.E.; Busch, T.M. Photodynamic therapy of disseminated non-small cell lung carcinoma in a murine model. Lasers Surg. Med. 2011, 43, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Rickter, E.; Yuan, M.; Wileyto, E.P.; Glatstein, E.; Yodh, A.; Busch, T.M. Effect of photosensitizer dose on fluence rate responses to photodynamic therapy. Photochem. Photobiol. 2007, 83, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Liu, Q.; Hamblin, M.R. A green fluorescent protein-expressing murine tumour but not its wild-type counterpart is cured by photodynamic therapy. Br. J. Cancer 2006, 94, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, S.; Molina, M.A.; Queralt, C.; de Aguirre, I.; Mayo, C.; Bertran-Alamillo, J.; Sanchez, J.J.; Gonzalez-Larriba, J.L.; Jimenez, U.; Isla, D.; et al. Detection of EGFR mutations with mutation-specific antibodies in stage IV non-small-cell lung cancer. J. Transl. Med. 2010, 8. [Google Scholar] [CrossRef] [PubMed]
- Angell-Petersen, E.; Spetalen, S.; Madsen, S.J.; Sun, C.H.; Peng, Q.; Carper, S.W.; Sioud, M.; Hirschberg, H. Influence of light fluence rate on the effects of photodynamic therapy in an orthotopic rat glioma model. J. Neurosurg. 2006, 104, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, J.H.; Macrobert, A.J.; Bown, S.G. The role of oxygen monitoring during photodynamic therapy and its potential for treatment dosimetry. Photochem. Photobiol. Sci. 2007, 6, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Yamamoto, S.; Hirano, T.; Li, S.; Koide, M.; Kohno, E.; Okada, M.; Inenaga, C.; Tokuyama, T.; Yokota, N.; et al. Monitoring of singlet oxygen is useful for predicting the photodynamic effects in the treatment for experimental glioma. Clin. Cancer Res. 2006, 12, 7132–7139. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, I.; Dinh, T.A.; Yu, W.; Chang, Y.; Sherwood, M.E.; Hasan, T. Photoimmunotherapy and irradiance modulation reduce chemotherapy cycles and toxicity in a murine model for ovarian carcinomatosis: Perspective and results. Isr. J. Chem. 2012, 52, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.W.; Greco, W.R.; Bellnier, D.A.; Vaughan, L.; Henderson, B.W. Photodynamic therapy: A means to enhanced drug delivery to tumors. Cancer Res. 2003, 63, 8126–8131. [Google Scholar] [PubMed]
- Pirker, R. What is the best strategy for targeting EGF receptors in non-small-cell lung cancer? Future Oncol. 2015, 11, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, C.; Hagan, S.; Gallagher-Colombo, S.M.; Busch, T.M.; Cengel, K.A. Photodynamic therapy activated signaling from epidermal growth factor receptor and STAT3: Targeting survival pathways to increase PDT efficacy in ovarian and lung cancer. Cancer Biol. Ther. 2012, 13, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Weyergang, A.; Selbo, P.K.; Berg, K. Y1068 phosphorylation is the most sensitive target of disulfonated tetraphenylporphyrin-based photodynamic therapy on epidermal growth factor receptor. Biochem. Pharmacol. 2007, 74, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Kalka, K.; Mukhtar, H. In vitro and in vivo inhibition of epidermal growth factor receptor-tyrosine kinase pathway by photodynamic therapy. Oncogene 2001, 20, 2314–2317. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, R.; Gan, Y.Y.; Soo, K.C.; Olivo, M. Targeting EGFR with photodynamic therapy in combination with Erbitux enhances in vivo bladder tumor response. Mol. Cancer 2009, 8. [Google Scholar] [CrossRef] [PubMed]
- Weyergang, A.; Kaalhus, O.; Berg, K. Photodynamic targeting of EGFR does not predict the treatment outcome in combination with the EGFR tyrosine kinase inhibitor Tyrphostin AG1478. Photochem. Photobiol. Sci. 2008, 7, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Ji, H.T.; Chiang, P.C.; Chou, R.H.; Chang, W.S.; Chen, C.T. ALA-PDT results in phenotypic changes and decreased cellular invasion in surviving cancer cells. Lasers Surg. Med. 2009, 41, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jiang, F.; Katakowski, M.; Zhang, X.; Jiang, H.; Zhang, Z.G.; Chopp, M. Sensitization of cerebral tissue in nude mice with photodynamic therapy induces ADAM17/TACE and promotes glioma cell invasion. Cancer Lett. 2008, 265, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, K.C.; Nyati, M.K.; Ray, D.; Lawrence, T.S. EGFR targeted therapies and radiation: Optimizing efficacy by appropriate drug scheduling and patient selection. Pharmacol. Ther. 2015, 154, 67–77. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grossman, C.E.; Carter, S.L.; Czupryna, J.; Wang, L.; Putt, M.E.; Busch, T.M. Fluence Rate Differences in Photodynamic Therapy Efficacy and Activation of Epidermal Growth Factor Receptor after Treatment of the Tumor-Involved Murine Thoracic Cavity. Int. J. Mol. Sci. 2016, 17, 101. https://doi.org/10.3390/ijms17010101
Grossman CE, Carter SL, Czupryna J, Wang L, Putt ME, Busch TM. Fluence Rate Differences in Photodynamic Therapy Efficacy and Activation of Epidermal Growth Factor Receptor after Treatment of the Tumor-Involved Murine Thoracic Cavity. International Journal of Molecular Sciences. 2016; 17(1):101. https://doi.org/10.3390/ijms17010101
Chicago/Turabian StyleGrossman, Craig E., Shirron L. Carter, Julie Czupryna, Le Wang, Mary E. Putt, and Theresa M. Busch. 2016. "Fluence Rate Differences in Photodynamic Therapy Efficacy and Activation of Epidermal Growth Factor Receptor after Treatment of the Tumor-Involved Murine Thoracic Cavity" International Journal of Molecular Sciences 17, no. 1: 101. https://doi.org/10.3390/ijms17010101
APA StyleGrossman, C. E., Carter, S. L., Czupryna, J., Wang, L., Putt, M. E., & Busch, T. M. (2016). Fluence Rate Differences in Photodynamic Therapy Efficacy and Activation of Epidermal Growth Factor Receptor after Treatment of the Tumor-Involved Murine Thoracic Cavity. International Journal of Molecular Sciences, 17(1), 101. https://doi.org/10.3390/ijms17010101