Regulatory System for Stem/Progenitor Cell Niches in the Adult Rodent Pituitary
Abstract
:1. Introduction
2. Pituitary Stem/Progenitor Cells and Their Niches
2.1. Identification of Pituitary Stem/Progenitor Cells
2.1.1. Side-Population Cells
2.1.2. SOX2+-Cells
2.1.3. Calcium-Binding Protein B (S100β+)-Cells
2.2. Construction of the Two Types of Pituitary Stem/Progenitor Cell Niche
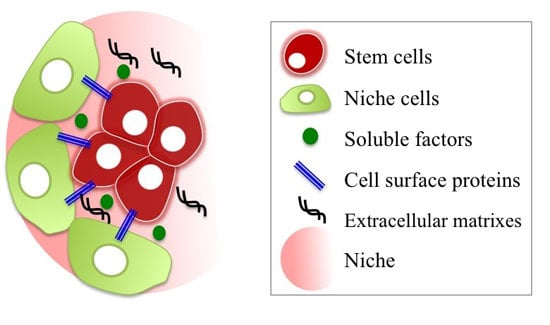
2.2.1. Stem/Progenitor Cell Niche
2.2.2. Two Types of Niche Constructed in the Adult Pituitary


| Signaling Types | Gene Symbol | Gene Title | Description | Localization and/or Expression in the | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCL-AL | MCL-IL | SOX2+-Cell Clusters | Non-SOX2+-Cell | S100β+-Cell | SP | Analyzed Species | |||||
| Soluble factor signaling | Fgfr | basic fibroblast growth factor receptor | Receptor for growth factor | – | – | – | – | – | c | m | [20] |
| Egfr | epidermal growth factor receptor | Receptor for growth factor | – | – | – | – | – | c | m | [20] | |
| Lifr | leukemia inhibitory factor receptor | Receptor for growth factor | – | – | – | – | – | c | m | [20] | |
| Ntn | Neurturin | Growth factor | – | – | – | a | – | – | r, h | [44] | |
| Gfra2 | GDNF receptor α 2 | Receptor for growth factor | a | a | – | – | – | – | m, r, h | [44] | |
| Cxcl12 (Sdf1) | Stromal cell-derived factor-1 | Chemokine | – | – | – | – | a, b, c, d | c | m, r | [42,45] | |
| Cxcr4 | C-X-C chemokine receptor type 4 | Receptor for Chemokine | – | – | – | a, b, c, d | a, b, c, d | c | m, r | [20,45,46] | |
| Juxtacrine signaling | Notch1 | Notch receptor 1 | Receptor for Notch signaling | b | b | b | – | b, c | c | m, r | [18,20,47] |
| Notch2 | Notch receptor 2 | Receptor for Notch signaling | b | b | b | – | b, c | c | m, r | [18,20,47] | |
| Notch3 | Notch receptor 3 | Receptor for Notch signaling | – | – | – | – | – | c | m | [18,20] | |
| Notch4 | Notch receptor 4 | Receptor for Notch signaling | – | – | – | – | – | c | m | [18,20] | |
| Jag1 | Jagged1 | Ligand for Notch signaling | b | b | b | – | b, c | c | m, r | [20,47] | |
| Jag2 | Jagged2 | Ligand for Notch signaling | – | b | – | b (in the IL) | – | – | r | [47] | |
| Dll4 | Delta-like protein 4 | Ligand for Notch signaling | – | – | – | – | – | c | m | [20] | |
| Efn-B2 | Ephrin-B2 | Ligand for ephrin/Eph signaling | a | a | a | – | a | c | m, r | [42,48] | |
| ECM-to-cell signaling | Itga1 | Integrin, α1 | Linkage of the ECM to the cells | – | – | – | c | c | – | r | [49] |
| Itga3 | Integrin, α3 | Linkage of the ECM to the cells | – | – | – | c | c | – | r | [49] | |
| Itga6 | Integrin, α6 | Linkage of the ECM to the cells | – | – | – | c | c | – | r | [49] | |
| Itgb1 | Integrin, β1 | Linkage of the ECM to the cells | – | – | – | c | a, c | – | r | [49] | |
| Lama5 | Laminin, α5 | ECM | b | b | b | – | – | – | r | [50] | |
| Sdc4 | Syndecan 4 | Transmembrane proteoglycan | – | – | – | c | c, d | – | r | [51] | |
| Dcn | Decorin | SLRPs | – | – | – | – | b | – | r | [52] | |
| Bgn | Biglycan | SLRPs | – | – | – | – | b | – | r | [52] | |
| Fmod | Fibromodulin | SLRPs | – | – | – | – | b | – | r | [52] | |
| Lum | Lumican | SLRPs | – | – | – | – | b | – | r | [52] | |
| Prelp | Proline/arginine-rich end leucine-rich repeat protein | SLRPs | – | – | – | – | b | – | r | [52] | |
| Ogn | Osteoglycan | SLRPs | – | – | – | – | b | – | r | [52] | |
3. Candidates for Regulator of Pituitary Stem/Progenitor Cell Niches
3.1. Soluble Factor Signaling
3.1.1. Growth Factor Signaling
3.1.2. Neurturin/Glial Cell-Line Derived Neurotrophic Factor (GDNF) Receptorα2 (GFRα2)/Co-Receptor of the Tyrosine Kinase (RET) Signaling
3.1.3. CXCL12/CXCR4 Signaling
3.2. Cell Surface Factor Signaling
3.2.1. Notch and Its Ligand
3.2.2. Ephrin and Eph
3.3. Extracellular Matrixes (ECMs)
3.3.1. ECMs and Integrins
3.3.2. ECMs in S100β+-Cells of the Pituitary
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, X.; Gleiberman, A.S.; Rosenfeld, M.G. Molecular physiology of pituitary development: Signaling and transcriptional networks. Physiol. Rev. 2007, 87, 933–963. [Google Scholar] [CrossRef] [PubMed]
- McNicol, M.A. A study of intermediate lobe differentiation in the human pituitary gland. J. Pathol. 1986, 150, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, F.; Harumiya, K.; Ishikawa, H.; Otsuka, Y. Differentiation of isolated chromophobes into acidophils or basophils when transplanted into the hypophysiotrophic area of hypothalamus. Endocrinol. Jpn. 1969, 16, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Castinetti, F.; Davis, S.W.; Brue, T.; Camper, S.A. Pituitary stem cell update and potential implications for treating hypopituitarism. Endocr. Rev. 2011, 32, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Nolan, L.A.; Levy, A. A population of non-luteinising hormone/non-adrenocorticotrophic hormone-positive cells in the male rat anterior pituitary responds mitotically to both gonadectomy and adrenalectomy. J. Neuroendocrinol. 2006, 18, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Andoniadou, C.L.; Matsushima, D.; Mousavy Gharavy, S.N.; Signore, M.; Mackintosh, A.I.; Schaeffer, M.; Gaston-Massuet, C.; Mollard, P.; Jacques, T.S.; le Tissier, P.; et al. SOX2+ stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell 2013, 13, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Fauquier, T.; Rizzoti, K.; Dattani, M.; Lovell-Badge, R.; Robinson, I.C. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc. Natl. Acad. Sci. USA 2008, 105, 2907–2912. [Google Scholar] [CrossRef] [PubMed]
- Rizzoti, K.; Akiyama, H.; Lovell-Badge, R. Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell 2013, 13, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: Mechanism and applications. Science 2013, 340, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Iacovitti, L. Classic and novel stem cell niches in brain homeostasis and repair. Brain Res. 2015, 1628, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Solanas, G.; Benitah, S.A. Regenerating the skin: A task for the heterogeneous stem cell pool and surrounding niche. Nat. Rev. Mol. Cell Biol. 2013, 14, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Gucciardo, E.; Sugiyama, N.; Lehti, K. Eph- and ephrin-dependent mechanisms in tumor and stem cell dynamics. Cell. Mol. Life Sci. 2014, 71, 3685–3710. [Google Scholar] [CrossRef] [PubMed]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hersmus, N.; van Duppen, V.; Caesens, P.; Denef, C.; Vankelecom, H. The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology 2005, 146, 3985–3998. [Google Scholar] [CrossRef] [PubMed]
- Goodell, M.A.; Brose, K.; Paradis, G.; Conner, A.S.; Mulligan, R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996, 183, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Crabbe, A.; van Duppen, V.; Vankelecom, H. The notch signaling system is present in the postnatal pituitary: Marked expression and regulatory activity in the newly discovered side population. Mol. Endocrinol. 2006, 20, 3293–3307. [Google Scholar] [CrossRef] [PubMed]
- Pastrana, E.; Silva-Vargas, V.; Doetsch, F. Eyes wide open: A critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 2011, 8, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gremeaux, L.; Fu, Q.; Liekens, D.; van Laere, S.; Vankelecom, H. Pituitary progenitor cells tracked down by side population dissection. Stem Cells 2009, 27, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kato, T.; Yako, H.; Susa, T.; Cai, L.-Y.; Osuna, M.; Inoue, K.; Kato, Y. Significant quantitative and qualitative transition in pituitary stem/progenitor cells occurs during the postnatal development of the rat anterior pituitary. J. Neuroendocrinol. 2011, 23, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Devnath, S.; Inoue, K. An insight to pituitary folliculo-stellate cells. J. Neuroendocrinol. 2008, 20, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Soji, T.; Sirasawa, N.; Kurono, C.; Yashiro, T.; Herbert, D.C. Immunohistochemical study of the post-natal development of the folliculo-stellate cells in the rat anterior pituitary gland. Tissue Cell 1994, 26, 1–8. [Google Scholar] [CrossRef]
- Ferrara, N.; Schweigerer, L.; Neufeld, G.; Mitchell, R.; Gospodarowicz, D. Pituitary follicular cells produce basic fibroblast growth factor. Proc. Natl. Acad. Sci. USA 1987, 84, 5773–5777. [Google Scholar] [CrossRef] [PubMed]
- Vankelecom, H.; Carmeliet, P.; van Damme, J.; Billiau, A.; Denef, C. Production of interleukin-6 by folliculo-stellate cells of the anterior pituitary gland in a histiotypic cell aggregate culture system. Neuroendocrinology 1989, 49, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Gospodarowicz, D.; Lau, K. Pituitary follicular cells secrete both vascular endothelial growth factor and follistatin. Biochem. Biophys. Res. Commun. 1989, 165, 292–298. [Google Scholar] [CrossRef]
- Findell, P.R.; Weiner, R.I. Bovine pituitary folliculo-stellate cells have beta-adrenergic receptors positively coupled to adenosine 3′,5′-cyclic monophosphate production. Endocrinology 1988, 123, 2454–2461. [Google Scholar] [CrossRef] [PubMed]
- Sudo, T.; Sakuma, Y.; Kato, M. Bradykinin and angiotensin II-induced [ca2+]i rise in cultured rat pituitary folliculo-stellate cells. J. Neuroendocrinol. 2001, 13, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Vigh, S.; Arimura, A.; Gottschall, P.E.; Kitada, C.; Somogyvari-Vigh, A.; Childs, G.V. Cytochemical characterization of anterior pituitary target cells for the neuropeptide, pituitary adenylate cyclase activating polypeptide (PACAP), using biotinylated ligands. Peptides 1993, 14, 59–65. [Google Scholar] [CrossRef]
- Prummel, M.F.; Brokken, L.J.; Meduri, G.; Misrahi, M.; Bakker, O.; Wiersinga, W.M. Expression of the thyroid-stimulating hormone receptor in the folliculo-stellate cells of the human anterior pituitary. J. Clin. Endocrinol. Metab. 2000, 85, 4347–4353. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Ishibashi, Y.; Sakamoto, Y.; Kitamura, K.; Kubo, M.; Sakai, T.; Inoue, K. The glycoproteins that occur in the colloids of senescent porcine pituitary glands are clusterin and glycosylated albumin fragments. Biochem. Biophys. Res. Commun. 1997, 234, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Soji, T.; Herbert, D.C. Intercellular communication between rat anterior pituitary cells. Anat. Rec. 1989, 224, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Allaerts, W.; Vankelecom, H. History and perspectives of pituitary folliculo-stellate cell research. Eur. J. Endocrinol. 2005, 153, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Kanno, N.; Yoshida, S.; Ueharu, H.; Chen, M.; Yako, H.; Shibuya, S.; Sekita, M.; Tsuda, M.; Mitsuishi, H.; et al. GFP-expressing S100β-positive cells of the rat anterior pituitary differentiate into hormone-producing cells. Cell Tissue Res. 2014, 357, 767–779. [Google Scholar] [CrossRef] [PubMed]
- De Cuevas, M.; Matunis, E.L. The stem cell niche: Lessons from the drosophila testis. Development 2011, 138, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; van den Born, M.; Barker, N.; Shroyer, N.F.; van de Wetering, M.; Clevers, H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011, 469, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Goritz, C.; Catchpole, T.; Henkemeyer, M.; Frisen, J. EphB signaling controls lineage plasticity of adult neural stem cell niche cells. Cell Stem Cell 2010, 7, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Gremeaux, L.; Fu, Q.; Chen, J.; Vankelecom, H. Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells Dev. 2012, 21, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Kato, T.; Higuchi, M.; Yoshida, S.; Yako, H.; Kanno, N.; Kato, Y. Coxsackievirus and adenovirus receptor-positive cells compose the putative stem/progenitor cell niches in the marginal cell layer and parenchyma of the rat anterior pituitary. Cell Tissue Res. 2013, 354, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kato, T.; Susa, T.; Cai, L.-Y.; Nakayama, M.; Kato, Y. PROP1 coexists with SOX2 and induces PIT1-commitment cells. Biochem. Biophys. Res. Commun. 2009, 385, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Hotta, Y.; Honda, T.; Naito, M.; Kuwano, R. Developmental distribution of coxsackie virus and adenovirus receptor localized in the nervous system. Brain Res. Dev. Brain Res. 2003, 143, 1–13. [Google Scholar] [CrossRef]
- Vankelecom, H. Pituitary stem/progenitor cells: Embryonic players in the adult gland? Eur. J. Neurosci. 2010, 32, 2063–2081. [Google Scholar] [CrossRef] [PubMed]
- Vankelecom, H. Pituitary stem cells drop their mask. Curr. Stem Cell Res. Ther. 2012, 7, 36–71. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lavandeira, M.; Quereda, V.; Flores, I.; Saez, C.; Diaz-Rodriguez, E.; Japon, M.A.; Ryan, A.K.; Blasco, M.A.; Dieguez, C.; Malumbres, M.; et al. A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PLoS ONE 2009, 4, e4815. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, K.; Ilmiawati, C.; Fujiwara, K.; Tsukada, T.; Kikuchi, M.; Yashiro, T. Expression of chemokine CXCL12 and its receptor CXCR4 in folliculostellate (FS) cells of the rat anterior pituitary gland: The CXCL12/CXCR4 axis induces interconnection of fs cells. Endocrinology 2012, 153, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, J.M.; Lee, E.J. Functional expression of CXCR4 in somatotrophs: CXCL12 activates GH gene, GH production and secretion, and cellular proliferation. J. Endocrinol. 2008, 199, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Tando, Y.; Fujiwara, K.; Yashiro, T.; Kikuchi, M. Localization of notch signaling molecules and their effect on cellular proliferation in adult rat pituitary. Cell Tissue Res. 2013, 351, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kato, T.; Chen, M.; Higuchi, M.; Ueharu, H.; Nishimura, N.; Kato, Y. Localization of a juxtacrine factor ephrin-B2 in the pituitary stem/progenitor cell niches throughout life. Cell Tissue Res. 2015, 359, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, K.; Kikuchi, M.; Kusumoto, K.; Fujiwara, K.; Kouki, T.; Kawanishi, K.; Yashiro, T. Living-cell imaging of transgenic rat anterior pituitary cells in primary culture reveals novel characteristics of folliculo-stellate cells. J. Endocrinol. 2010, 204, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Ramadhani, D.; Tsukada, T.; Fujiwara, K.; Azuma, M.; Kikuchi, M.; Yashiro, T. Changes in laminin chain expression in pre- and postnatal rat pituitary gland. Acta Histochem. Cytochem. 2014, 47, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, K.; Kouki, T.; Fujiwara, K.; Tsukada, T.; Ly, F.; Kikuchi, M.; Yashiro, T. Expression of the proteoglycan syndecan-4 and the mechanism by which it mediates stress fiber formation in folliculostellate cells in the rat anterior pituitary gland. J. Endocrinol. 2012, 214, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, K.; Syaidah, R.; Fujiwara, K.; Tsukada, T.; Ramadhani, D.; Jindatip, D.; Kikuchi, M.; Yashiro, T. Expression of small leucine-rich proteoglycans in rat anterior pituitary gland. Cell Tissue Res. 2013, 351, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Buczacki, S.J.; Zecchini, H.I.; Nicholson, A.M.; Russell, R.; Vermeulen, L.; Kemp, R.; Winton, D.J. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 2013, 495, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Korinek, V.; Barker, N.; Moerer, P.; van Donselaar, E.; Huls, G.; Peters, P.J.; Clevers, H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking TCF-4. Nat. Genet. 1998, 19, 379–383. [Google Scholar] [PubMed]
- Wong, V.W.; Stange, D.E.; Page, M.E.; Buczacki, S.; Wabik, A.; Itami, S.; van de Wetering, M.; Poulsom, R.; Wright, N.A.; Trotter, M.W.; et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat. Cell Biol. 2012, 14, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinet, L.; Rodilla, V.; Liu, Z.; Chen, S.; Koch, U.; Espinosa, L.; Kaestner, K.H.; Kopan, R.; Lewis, J.; Radtke, F. Dll1- and Dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 2011, 140, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Henderson, J.T.; Beghtel, H.; van den Born, M.M.; Sancho, E.; Huls, G.; Meeldijk, J.; Robertson, J.; van de Wetering, M.; Pawson, T.; et al. β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/EphrinB. Cell 2002, 111, 251–263. [Google Scholar] [CrossRef]
- Dennis, K.; Nguyen, J.; Presutti, R.; DeAngelis, C.; Tsao, M.; Danjoux, C.; Barnes, E.; Sahgal, A.; Holden, L.; Jon, F.; et al. Prophylaxis of radiotherapy-induced nausea and vomiting in the palliative treatment of bone metastases. Support Care Cancer 2012, 20, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Childs, G.V.; Armstrong, J. Sites of epidermal growth factor synthesis and action in the pituitary: Paracrine and autocrine interactions. Clin. Exp. Pharmacol. Physiol. 2001, 28, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.; Melmed, S. Pituitary cytokine and growth factor expression and action. Endocr. Rev. 1997, 18, 206–228. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.C.; Childs, G.V. Nerve growth factor and its receptor in the anterior pituitary. Endocrinology 1994, 135, 1689–1696. [Google Scholar] [PubMed]
- Denef, C. Paracrine control of lactotrope proliferation and differentiation. Trends Endocrinol. Metab. 2003, 14, 188–195. [Google Scholar] [CrossRef]
- Airaksinen, M.S.; Saarma, M. The GDNF family: Signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002, 3, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Inoue, K.; Takashima, S.; Takehashi, M.; Ogonuki, N.; Morimoto, H.; Nagasawa, T.; Ogura, A.; Shinohara, T. Reconstitution of mouse spermatogonial stem cell niches in culture. Cell Stem Cell 2012, 11, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Paratcha, G.; Ledda, F. GDNF and GFRα: A versatile molecular complex for developing neurons. Trends Neurosci. 2008, 31, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, K.S.; Schaumburg, C.; Strieter, R.; Kane, J.; Lane, T.E. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through cxcr4 in a viral model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2010, 107, 11068–11073. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cheng, G.; Hao, M.; Zheng, J.; Zhou, X.; Zhang, J.; Taichman, R.S.; Pienta, K.J.; Wang, J. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010, 29, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Kulesa, P.M.; Gammill, L.S. Neural crest migration: Patterns, phases and signals. Dev. Biol. 2010, 344, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Ara, T.; Tokoyoda, K.; Sugiyama, T.; Egawa, T.; Kawabata, K.; Nagasawa, T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 2003, 19, 257–267. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Tokoyoda, K.; Egawa, T.; Sugiyama, T.; Choi, B.I.; Nagasawa, T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity 2004, 20, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, K.; Fujiwara, K.; Tsukada, T.; Yako, H.; Tateno, K.; Hasegawa, R.; Takegami, S.; Osako, S.; Yashiro, T.; Kato, T.; et al. Expression of slug in S100β protein-positive cells of the postnatal developing rat anterior pituitary gland. Cell Tissue Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ables, J.L.; Breunig, J.J.; Eisch, A.J.; Rakic, P. Not(ch) just development: Notch signalling in the adult brain. Nat. Rev. Neurosci. 2011, 12, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, J.; Tollkuhn, J.; Ohsawa, R.; Bresnick, E.H.; Guillemot, F.; Kageyama, R.; Rosenfeld, M.G. Sustained notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 2006, 20, 2739–2753. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tollkuhn, J.; Taylor, H.; Rosenfeld, M.G. Notch-dependent pituitary SOX2+ stem cells exhibit a timed functional extinction in regulation of the postnatal gland. Stem Cell Rep. 2015, 5, 1196–1209. [Google Scholar] [CrossRef] [PubMed]
- Nantie, L.B.; Himes, A.D.; Getz, D.R.; Raetzman, L.T. Notch signaling in postnatal pituitary expansion: Proliferation, progenitors, and cell specification. Mol. Endocrinol. 2014, 28, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Murai, K.K.; Pasquale, E.B. Eph’ective signaling: Forward, reverse and crosstalk. J. Cell Sci. 2003, 116, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, E.B. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005, 6, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.L. Eph receptors and ephrins. Stem Cells 2000, 18, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Daar, I.O. Non-SH2/PDZ reverse signaling by ephrins. Semin. Cell Dev. Biol. 2012, 23, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, J.; Genander, M.; Halford, M.M.; Anneren, C.; Sondell, M.; Chumley, M.J.; Silvany, R.E.; Henkemeyer, M.; Frisen, J. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell 2006, 125, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Hafner, C.; Meyer, S.; Hagen, I.; Becker, B.; Roesch, A.; Landthaler, M.; Vogt, T. Ephrin-B reverse signaling induces expression of wound healing associated genes in IEC-6 intestinal epithelial cells. World J. Gastroenterol. 2005, 11, 4511–4518. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Hafner, C.; Guba, M.; Flegel, S.; Geissler, E.K.; Becker, B.; Koehl, G.E.; Orso, E.; Landthaler, M.; Vogt, T. Ephrin-B2 overexpression enhances integrin-mediated ECM-attachment and migration of B16 melanoma cells. Int. J. Oncol. 2005, 27, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Nakada, M.; Anderson, E.M.; Demuth, T.; Nakada, S.; Reavie, L.B.; Drake, K.L.; Hoelzinger, D.B.; Berens, M.E. The phosphorylation of ephrin-B2 ligand promotes glioma cell migration and invasion. Int. J. Cancer 2010, 126, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Ashton, R.S.; Conway, A.; Pangarkar, C.; Bergen, J.; Lim, K.I.; Shah, P.; Bissell, M.; Schaffer, D.V. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat. Neurosci. 2012, 15, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Durbeej, M. Laminins. Cell Tissue Res. 2010, 339, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Kazanis, I.; ffrench-Constant, C. Extracellular matrix and the neural stem cell niche. Dev. Neurobiol. 2011, 71, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Wang, Y.; Kokovay, E.; Lin, G.; Chuang, S.M.; Goderie, S.K.; Roysam, B.; Temple, S. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell 2008, 3, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Staquicini, F.I.; Dias-Neto, E.; Li, J.; Snyder, E.Y.; Sidman, R.L.; Pasqualini, R.; Arap, W. Discovery of a functional protein complex of netrin-4, laminin gamma1 chain, and integrin α6β1 in mouse neural stem cells. Proc. Natl. Acad. Sci. USA 2009, 106, 2903–2908. [Google Scholar] [CrossRef] [PubMed]
- Kazanis, I.; Lathia, J.D.; Vadakkan, T.J.; Raborn, E.; Wan, R.; Mughal, M.R.; Eckley, D.M.; Sasaki, T.; Patton, B.; Mattson, M.P.; et al. Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals. J. Neurosci. 2010, 30, 9771–9781. [Google Scholar] [CrossRef] [PubMed]
- Itakura, E.; Odaira, K.; Yokoyama, K.; Osuna, M.; Hara, T.; Inoue, K. Generation of transgenic rats expressing green fluorescent protein in S-100β-producing pituitary folliculo-stellate cells and brain astrocytes. Endocrinology 2007, 148, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, K.; Fujiwara, K.; Ilmiawati, C.; Kikuchi, M.; Tsukada, T.; Kouki, T.; Yashiro, T. Caveolin 3-mediated integrin β1 signaling is required for the proliferation of folliculostellate cells in rat anterior pituitary gland under the influence of extracellular matrix. J. Endocrinol. 2011, 210, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ramadhani, D.; Tsukada, T.; Fujiwara, K.; Horiguchi, K.; Kikuchi, M.; Yashiro, T. Laminin isoforms and laminin-producing cells in rat anterior pituitary. Acta Histochem. Cytochem. 2012, 45, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Rodin, S.; Domogatskaya, A.; Strom, S.; Hansson, E.M.; Chien, K.R.; Inzunza, J.; Hovatta, O.; Tryggvason, K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 2010, 28, 611–615. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, S.; Kato, T.; Kato, Y. Regulatory System for Stem/Progenitor Cell Niches in the Adult Rodent Pituitary. Int. J. Mol. Sci. 2016, 17, 75. https://doi.org/10.3390/ijms17010075
Yoshida S, Kato T, Kato Y. Regulatory System for Stem/Progenitor Cell Niches in the Adult Rodent Pituitary. International Journal of Molecular Sciences. 2016; 17(1):75. https://doi.org/10.3390/ijms17010075
Chicago/Turabian StyleYoshida, Saishu, Takako Kato, and Yukio Kato. 2016. "Regulatory System for Stem/Progenitor Cell Niches in the Adult Rodent Pituitary" International Journal of Molecular Sciences 17, no. 1: 75. https://doi.org/10.3390/ijms17010075
APA StyleYoshida, S., Kato, T., & Kato, Y. (2016). Regulatory System for Stem/Progenitor Cell Niches in the Adult Rodent Pituitary. International Journal of Molecular Sciences, 17(1), 75. https://doi.org/10.3390/ijms17010075