Ocular Albinism Type 1 Regulates Melanogenesis in Mouse Melanocytes
Abstract
:1. Introduction
2. Results
2.1. Expression Profile of Ocular Albinism Type 1 (OA1) mRNA in Mice Skin Samples
2.2. Protein Expression of OA1 in Mice Skin Samples
2.3. Distribution and Expression of OA1 in Mice Skin Samples
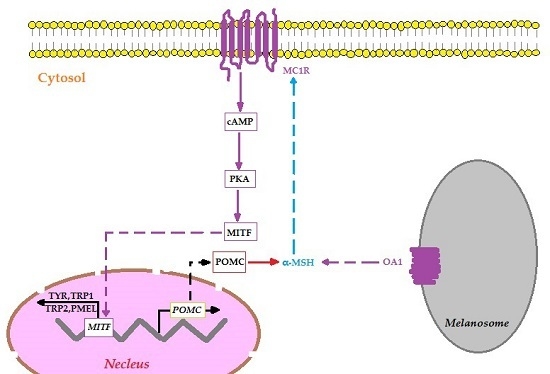
2.4. OA1 Controls Not Only Melanosome Structural Protein but Also the Melanin Synthesis in an MITF-Dependent Fashion
3. Discussion
4. Materials and Methods
4.1. Antibody
4.2. Experimental Animals and Sample Collection
4.3. Cell Culture and Transfection
4.4. Melanin Content Measurement
4.5. RNA Extraction and qRT-PCR Analysis
4.6. Western Blotting
4.7. Localization of OA1 by Immunofluorescence Staining
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seiji, M.; Fitzpatrick, T.B.; Simpson, R.T.; Birbeck, M.S. Chemical composition and terminology of specialized organelles (melanosomes and melanin granules) in mammalian melanocytes. Nature 1963, 197, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J. Biogenesis of pigment granules: A sensitive way to regulate melanocyte function. J. Dermatol. Sci. 2005, 37, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.; Valencia, J.C.; Hu, Z.Z.; Watabe, H.; Yamaguchi, H.; Mangini, N.J.; Huang, H.; Canfield, V.A.; Cheng, K.C.; Yang, F.; et al. Proteomic and bioinformatic characterization of the biogenesis and function of melanosomes. J. Proteome Res. 2006, 5, 3135–3144. [Google Scholar] [CrossRef] [PubMed]
- Basrur, V.; Yang, F.; Kushimoto, T.; Higashimoto, Y.; Yasumoto, K.; Valencia, J.; Muller, J.; Vieira, W.D.; Watabe, H.; Shabanowitz, J.; et al. Proteomic analysis of early melanosomes: Identification of novel melanosomal proteins. J. Proteome Res. 2003, 2, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Korner, A.; Pawelek, J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science 1982, 217, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- McKay, B.S.; Schwartz, S.G. Pigmentation and macular degeneration: Is there a role for GPR143? J. Ocul. Pharmacol. Ther. 2016, 32, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Simoes, S.; Raposo, G. The ocular albinism type 1 (OA1) GPCR is ubiquitinated and its traffic requires endosomal sorting complex responsible for transport (ESCRT) function. Proc. Natl. Acad. Sci. USA 2011, 108, 11906–11911. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.T.; Schiaffino, M.V.; Renieri, A.; de Nigris, F.; Galli, L.; Bruttini, M.; Gebbia, M.; Bergen, A.A.; Lewis, R.A.; Ballabio, A. Cloning of the gene for ocular albinism type 1 from the distal short arm of the X chromosome. Nat. Genet. 1995, 10, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, M.V.; Baschirotto, C.; Pellegrini, G.; Montalti, S.; Tacchetti, C.; De Luca, M.; Ballabio, A. The ocular albinism type 1 gene product is a membrane glycoprotein localized to melanosomes. Proc. Natl. Acad. Sci. USA 1996, 93, 9055–9060. [Google Scholar] [CrossRef] [PubMed]
- Sone, M.; Orlow, S.J. The ocular albinism type 1 gene product, OA1, spans intracellular membranes 7 times. Exp. Eye Res. 2007, 85, 806–816. [Google Scholar] [CrossRef] [PubMed]
- D’Addio, M.; Pizzigoni, A.; Bassi, M.T.; Baschirotto, C.; Valetti, C.; Incerti, B.; Clementi, M.; de Luca, M.; Ballabio, A.; Schiaffino, M.V. Defective intracellular transport and processing of OA1 is a major cause of ocular albinism type 1. Hum. Mol. Genet. 2000, 9, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, M.V.; d’Addio, M.; Alloni, A.; Baschirotto, C.; Valetti, C.; Cortese, K.; Puri, C.; Bassi, M.T.; Colla, C.; de Luca, M.; et al. Ocular albinism: Evidence for a defect in an intracellular signal transduction system. Nat. Genet. 1999, 23, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Innamorati, G.; Piccirillo, R.; Bagnato, P.; Palmisano, I.; Schiaffino, M.V. The melanosomal/lysosomal protein OA1 has properties of a G protein-coupled receptor. Pigment Cell Res. 2006, 19, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Lopez, V.M.; Decatur, C.L.; Stamer, W.D.; Lynch, R.M.; McKay, B.S. L-DOPA is an endogenous ligand for OA1. PLoS Biol. 2008, 6, e236. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Naito, S.; Masukawa, D.; Kaneda, M.; Miyamoto, H.; Abe, T.; Yamashita, Y.; Endo, I.; Nakamura, F.; Goshima, Y. Expression of ocular albinism 1 (OA1), 3, 4-dihydroxy-L-phenylalanine (DOPA) receptor, in both neuronal and non-neuronal organs. Brain Res. 2015, 1602, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Masukawa, D.; Nakamura, F.; Koga, M.; Kamiya, M.; Chen, S.; Yamashita, N.; Arai, N.; Goshima, Y. Localization of ocular albinism-1 gene product GPR143 in the rat central nervous system. Neurosci. Res. 2014, 88, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Miyamoto, H.; Nakamura, F.; Masukawa, D.; Yamamoto, T.; Muraoka, H.; Kamiya, M.; Yamashita, N.; Suzuki, T.; Matsuzaki, S.; et al. The protein Ocular Albinism 1 is the orphan GPCR GPR143 and mediates depressor and bradycardic responses to DOPA in the nucleus tractus solitarii. Br. J. Pharmacol. 2014, 171, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, B.; Gopalakrishnan, C.; Purohit, R. In silico analysis of mirna-mediated gene regulation in OCA and OA genes. Cell Biochem. Biophys. 2014, 70, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Vetrini, F.; Auricchio, A.; Du, J.; Angeletti, B.; Fisher, D.E.; Ballabio, A.; Marigo, V. The microphthalmia transcription factor (MITF) controls expression of the ocular albinism type 1 gene: Link between melanin synthesis and melanosome biogenesis. Mol. Cell. Biol. 2004, 24, 6550–6559. [Google Scholar] [CrossRef] [PubMed]
- Vachtenheim, J.; Borovansky, J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Steingrimsson, E.; Copeland, N.G.; Jenkins, N.A. Melanocytes and the microphthalmia transcription factor network. Annu. Rev. Genet. 2004, 38, 365–411. [Google Scholar] [CrossRef] [PubMed]
- Falletta, P.; Bagnato, P.; Bono, M.; Monticone, M.; Schiaffino, M.V.; Bennett, D.C.; Goding, C.R.; Tacchetti, C.; Valetti, C. Melanosome-autonomous regulation of size and number: The OA1 receptor sustains PMEL expression. Pigment Cell Melanoma Res. 2014, 27, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Ujvari, A.; Aron, R.; Eisenhaure, T.; Cheng, E.; Parag, H.A.; Smicun, Y.; Halaban, R.; Hebert, D.N. Translation rate of human tyrosinase determines its N-linked glycosylation level. J. Biol. Chem. 2001, 276, 5924–5931. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, C.J.; Garratt, P.J.; Land, E.J.; Pavel, S.; Ramsden, C.A.; Riley, P.A.; Smit, N.P. Evidence of the indirect formation of the catecholic intermediate substrate responsible for the autoactivation kinetics of tyrosinase. J. Biol. Chem. 1997, 272, 26226–26235. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Imokawa, G.; Bennett, D.C.; Hearing, V.J. Tyrosinase stabilization by Tyrp1 (the brown locus protein). J. Biol. Chem. 1998, 273, 31801–31805. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.J.; Chambers, D.M.; Tsukamoto, K.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Hearing, V. A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. EMBO J. 1992, 11, 527–535. [Google Scholar] [PubMed]
- Tsukamoto, K.; Jackson, I.J.; Urabe, K.; Montague, P.M.; Hearing, V.J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992, 11, 519–526. [Google Scholar] [PubMed]
- Berson, J.F.; Theos, A.C.; Harper, D.C.; Tenza, D.; Raposo, G.; Marks, M.S. Proprotein convertase cleavage liberates a fibrillogenic fragment of a resident glycoprotein to initiate melanosome biogenesis. J. Cell Biol. 2003, 161, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Urabe, K.; Orlow, S.J.; Higashi, K.; Imokawa, G.; Kwon, B.S.; Potterf, B.; Hearing, V.J. The Pmel 17/silver locus protein. Characterization and investigation of its melanogenic function. J. Biol. Chem. 1994, 269, 29198–29205. [Google Scholar] [PubMed]
- Theos, A.C.; Berson, J.F.; Theos, S.C.; Herman, K.E.; Harper, D.C.; Tenza, D.; Sviderskaya, E.V.; Lamoreux, M.L.; Bennett, D.C.; Raposo, G.; et al. Dual loss of ER export and endocytic signals with altered melanosome morphology in the silver mutation of Pmel17. Mol. Biol. Cell 2006, 17, 3598–3612. [Google Scholar] [CrossRef] [PubMed]
- Hoashi, T.; Sato, S.; Yamaguchi, Y.; Passeron, T.; Tamaki, K.; Hearing, V.J. Glycoprotein nonmetastatic melanoma protein b, a melanocytic cell marker, is a melanosome-specific and proteolytically released protein. FASEB J. 2010, 24, 1616–1629. [Google Scholar] [CrossRef] [PubMed]
- Weterman, M.A.; Ajubi, N.; van Dinter, I.M.; Degen, W.G.; van Muijen, G.N.; Ruitter, D.J.; Bloemers, H.P. Nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int. J. Cancer 1995, 60, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Prota, G.; Hu, D.N.; Vincensi, M.R.; McCormick, S.A.; Napolitano, A. Characterization of melanins in human irides and cultured uveal melanocytes from eyes of different colors. Exp. Eye Res. 1998, 67, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Scriver, C.R.; Stanbury, J.B.; Wyngaarden, J.B.; Fredrickson, D.S. The Metabolic and Molecular Bases of Inherited Disease; McGraw-Hill: New York, NY, USA, 1997; pp. 843–844. [Google Scholar]
- Hearing, V.J. Unraveling the melanocyte. Am. J. Hum. Genet. 1993, 52, 1–7. [Google Scholar] [PubMed]
- Hearing, V.J. The melanosome: The perfect model for cellular responses to the environment. Pigment Cell Res. 2000, 13, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Ito, S. The IFPCS presidential lecture: A chemist’s view of melanogenesis. Pigment Cell Res. 2003, 16, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Human hair melanins: What we have learned and have not learned from mouse coat color pigmentation. Pigment Cell Melanoma Res. 2011, 24, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.; Hori, Y.; Toda, K.; Seiji, M. Melanin 1969: Some definitions and problems. Jpn. J. Dermatol. B 1969, 79, 278–282. [Google Scholar]
- Mayer, T.C. The migratory pathway of neural crest cells into the skin of mouse embryos. Dev. Biol. 1973, 34, 39–46. [Google Scholar] [CrossRef]
- Nishimura, E.K.; Yoshida, H.; Kunisada, T.; Nishikawa, S.I. Regulation of E- and P-cadherin expression correlated with melanocyte migration and diversification. Dev. Biol. 1999, 215, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Rawles, M.E. Origin of pigment cells from the neural crest in the mouse embryo. Physiol. Zool. 1947, 20, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, T. Histochemical survey of the distribution of the epidermal melanoblasts and melanocytes in the mouse during fetal and postnatal periods. Anat. Rec. 1984, 208, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, T.; Norris, D.A.; Johnson, T.W.; Zekman, T.; Dunscomb, N.; Bennion, S.D.; Jackson, R.L.; Morelli, J.G. DOPA-negative melanocytes in the outer root sheath of human hair follicles express premelanosomal antigens but not a melanosomal antigen or the melanosome-associated glycoproteins tyrosinase, TRP-1, and TRP-2. J. Investig. Dermatol. 1996, 106, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Sarin, K.Y.; Artandi, S.E. Aging, graying and loss of melanocyte stem cells. Stem Cell Rev. 2007, 3, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Plonka, P.M.; Schallreuter, K.U.; Paus, R.; Tobin, D.J. Hair follicle pigmentation. J. Investig. Dermatol. 2005, 124, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J.; Bystryn, J.C. Different populations of melanocytes are present in hair follicles and epidermis. Pigment Cell Res. 1996, 9, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shen, L.Y.; Wang, G.C. Role of hair follicles in the repigmentation of vitiligo. J. Investig. Dermatol. 1991, 97, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Ortonne, J.P.; Pelletier, N.; Chabanon, M.; Thivolet, J. Vitiligo and cutaneous epitheliomas. Ann. Dermatol. Venereol. 1978, 105, 1063–1064. [Google Scholar] [PubMed]
- Giordano, F.; Bonetti, C.; Surace, E.M.; Marigo, V.; Raposo, G. The ocular albinism type 1 (OA1) G-protein-coupled receptor functions with MART-1 at early stages of melanogenesis to control melanosome identity and composition. Hum. Mol. Genet. 2009, 18, 4530–4545. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, F.E.; Hambrick, G.W.; Green, W.R.; Iliff, W.J.; Stone, D.L. X-linked ocular albinism: An oculocutaneous macromelanosomal disorder. Arch. Ophthalmol. 1976, 94, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Cortese, K.; Giordano, F.; Surace, E.M.; Venturi, C.; Ballabio, A.; Tacchetti, C.; Marigo, V. The ocular albinism type 1 (OA1) gene controls melanosome maturation and size. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4358–4364. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, I.; Bagnato, P.; Palmigiano, A.; Innamorati, G.; Rotondo, G.; Altimare, D.; Venturi, C.; Sviderskaya, E.V.; Piccirillo, R.; Coppola, M.; et al. The ocular albinism type 1 protein, an intracellular G protein-coupled receptor, regulates melanosome transport in pigment cells. Hum. Mol. Genet. 2008, 17, 3487–3501. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, M.V. Signaling pathways in melanosome biogenesis and pathology. Int. J. Biochem. Cell Biol. 2010, 42, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, T.; Jolly, R.; Martin-Martin, B.; Seabra, M.C.; Piccirillo, R.; Schiaffino, M.V.; Futter, C.E. Expression of OA1 limits the fusion of a subset of MVBs with lysosomes—A mechanism potentially involved in the initial biogenesis of melanosomes. J. Cell Sci. 2013, 126, 5143–5152. [Google Scholar] [CrossRef] [PubMed]
- Busca, R.; Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000, 13, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Miller, A.J.; Widlund, H.R.; Horstmann, M.A.; Ramaswamy, S.; Fisher, D.E. MlANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am. J. Pathol. 2003, 163, 333–343. [Google Scholar] [CrossRef]
- Loftus, S.K.; Antonellis, A.; Matera, I.; Renaud, G.; Baxter, L.L.; Reid, D.; Wolfsberg, T.G.; Chen, Y.; Wang, C.; Prasad, M.K.; et al. Gpnmb is a melanoblast-expressed, MITF-dependent gene. Pigment Cell Melanoma Res. 2009, 22, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Incerti, B.; Cortese, K.; Pizzigoni, A.; Surace, E.M.; Varani, S.; Coppola, M.; Jeffery, G.; Seeliger, M.; Jaissle, G.; Bennett, D.C.; et al. OA1 knock-out: New insights on the pathogenesis of ocular albinism type 1. Hum. Mol. Genet. 2000, 9, 2781–2788. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, T.; O’Connor, M.N.; Seabra, M.C.; Cutler, D.F.; Futter, C.E. Regulation of melanosome number, shape and movement in the zebrafish retinal pigment epithelium by OA1 and PMEL. J. Cell Sci. 2015, 128, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.; Yokoyama, K.; Takahashi, K.; Tomita, Y.; Shibahara, S. Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes. J. Biol. Chem. 1997, 272, 503–509. [Google Scholar] [PubMed]
- Shi, Z.; Ji, K.; Yang, S.; Zhang, J.; Yao, J.; Dong, C.; Fan, R. Biological characteristics of mouse skin melanocytes. Tissue Cell 2016, 48, 114–120. [Google Scholar] [CrossRef] [PubMed]





| Primer Name | Primer Sequence 5’→3’ | PCR Production (bp) |
|---|---|---|
| Mus-OA1-F-XhoI | CCGCTCGAGGCCACCATGGCCTCCCCGCGCCT | 1218 |
| Mus-OA1-R-EcoRI | CGGAATTCTCAGAGTTCCCCCTGGGCTTG | |
| Mus-OA1-F | ATCAGGGCGTCGATCTGTTG | 193 |
| Mus-OA1-R | AGCAGGCCAAATGTCTGTTG | |
| Mus-MITF-F | AGGACCTTGAAAACCGACAG | 115 |
| Mus-MITF-R | GTGGATGGGATAAGGGAAAG | |
| Mus-TYR-F | ACTTACTCAGCCCAGCATCC | 109 |
| Mus-TYR-R | AGTGGTCCCTCAGGTGTTCC | |
| Mus-TRP1-F | CTTGGAGGTCCGTGTATTTG | 223 |
| Mus-TRP1-R | GACCGCATCAGTGAAAGTGT | |
| Mus-TRP2-F | CCAACGCTGATTAGTCGGA | 213 |
| Mus-TRP2-R | GAAGAAGGGAGGGCTGTCA | |
| Mus-GPNMB-F | GGGCATACATTCCCATCTCG | 215 |
| Mus-GPNMB-R | AGTGTTGTCCCCAAAGTTCCA | |
| Mus-PMEL-F | AGTGTTGTCCCCAAAGTTCCA | 171 |
| Mus-PMEL-R | AGGAGCGGGCTGTTTTCT | |
| Mus-β-actin-F | TTGCTGACAGGATGCAGAAG | 140 |
| Mus-β-actin-R | TTGCTGACAGGATGCAGAAG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Wang, H.; Liu, Y.; Zhao, B.; Zhao, Y.; Fan, R.; Wang, P.; Dong, C. Ocular Albinism Type 1 Regulates Melanogenesis in Mouse Melanocytes. Int. J. Mol. Sci. 2016, 17, 1596. https://doi.org/10.3390/ijms17101596
Chen T, Wang H, Liu Y, Zhao B, Zhao Y, Fan R, Wang P, Dong C. Ocular Albinism Type 1 Regulates Melanogenesis in Mouse Melanocytes. International Journal of Molecular Sciences. 2016; 17(10):1596. https://doi.org/10.3390/ijms17101596
Chicago/Turabian StyleChen, Tianzhi, Haidong Wang, Yu Liu, Bingling Zhao, Yuanyuan Zhao, Ruiwen Fan, Pengchao Wang, and Changsheng Dong. 2016. "Ocular Albinism Type 1 Regulates Melanogenesis in Mouse Melanocytes" International Journal of Molecular Sciences 17, no. 10: 1596. https://doi.org/10.3390/ijms17101596
APA StyleChen, T., Wang, H., Liu, Y., Zhao, B., Zhao, Y., Fan, R., Wang, P., & Dong, C. (2016). Ocular Albinism Type 1 Regulates Melanogenesis in Mouse Melanocytes. International Journal of Molecular Sciences, 17(10), 1596. https://doi.org/10.3390/ijms17101596