Rat Aquaporin-5 Is pH-Gated Induced by Phosphorylation and Is Implicated in Oxidative Stress
Abstract
:1. Introduction
2. Results
2.1. Subcellular Localization and Water Permeability of Rat AQP5 Expressed in Yeast
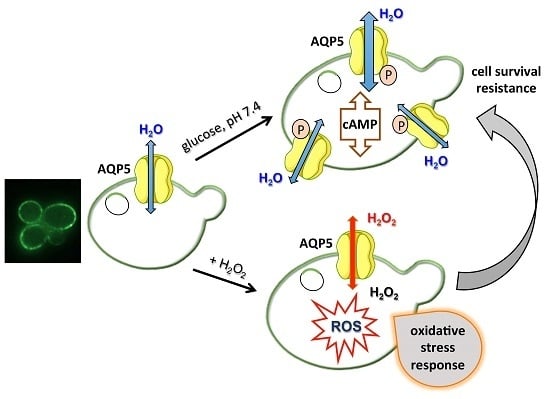
2.2. Effect of pH and Glucose-Stimulated Phosphorylation on Rat AQP5 Permeability
2.3. Hydrogen Peroxide Consumption
2.4. AQP5 Implication on Cell Oxidative Status
2.5. Yeast Sensitivity to Hydrogen Peroxide
3. Discussion
4. Materials and Methods
4.1. Yeast Strains and Growth Conditions
4.2. Cloning and Heterologous Expression of AQP5 in S. cerevisiae
4.3. AQP5 Subcellular Location by Fluorescence Microscopy
4.4. Cell Sampling and CFDA Loading
4.5. Cell Volume Measurements
4.6. Water Permeability Assays
4.7. External pH Dependence and In Vivo PKA Phosphorylation
4.8. Hydrogen Peroxide Consumption
4.9. Yeast Sensitivity Assays
4.10. Preparation of Cell Lysates for Colorimetric Assays
4.11. Catalase Activity Analysis
4.12. Determination of GSH Levels
4.13. Intracellular ROS Analysis
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations:
| AQP | Aquaporin |
| cAMP | Cyclic adenosine monophosphate |
| GSH | Glutathione |
| Ea | Activation energy |
| Pf | Osmotic permeability coefficient |
| PKA | Protein kinase A |
| ROS | Reactive oxygen species |
References
- Carbrey, J.M.; Agre, P. Discovery of the aquaporins and development of the field. In Handbook of Experimental Pharmacology; Beitz, E., Ed.; Springer: Berlin/Heidelberg, Gemany, 2009; Volume 190, pp. 3–28. [Google Scholar]
- Madeira, A.; Moura, T.F.; Soveral, G. Detecting aquaporin function and regulation. Front. Chem. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Aquaporins in clinical medicine. Annu. Rev. Med. 2012, 63, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Soveral, G.; Nielsen, S.; Casini, A. Aquaporins in Health and Disease: New Molecular Targets for Drug Discovery; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; Bryla, A.; Perret, J. Aquaporins in salivary glands: From basic research to clinical applications. Int. J. Mol. Sci. 2016, 17, 166. [Google Scholar] [CrossRef] [PubMed]
- Direito, I.; Madeira, A.; Brito, M.A.; Soveral, G. Aquaporin-5: From structure to function and dysfunction in cancer. Cell. Mol. Life Sci. 2016, 73, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Lee, J.; Kim, M.S.; Jang, S.J.; Sidransky, D.; Moon, C. The effect of aquaporin 5 overexpression on the RAS signaling pathway. Biochem. Biophys. Res. Commun. 2008, 367, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Woo, J.; Kim, M.J.; Kang, S.K.; Kim, M.S.; Lee, J.; Lee, S.K.; Gong, G.; Kim, Y.H.; Soria, J.C.; et al. Expression of aquaporin 5 (AQP5) promotes tumor invasion in human non-small cell lung cancer. PLoS ONE 2008, 3, e2162. [Google Scholar] [CrossRef]
- Kang, S.K.; Chae, Y.K.; Woo, J.; Kim, M.S.; Park, J.C.; Lee, J.; Soria, J.C.; Jang, S.J.; Sidransky, D.; Moon, C. Role of human aquaporin 5 in colorectal carcinogenesis. Am. J. Pathol. 2008, 173, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S. Key roles of aquaporins in tumor biology. Biochim. Biophys. Acta 2015, 1848, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.; Cadenas, E. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000, 475, 121–126. [Google Scholar] [CrossRef]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef] [PubMed]
- Vieceli Dalla Sega, F.; Zambonin, L.; Fiorentini, D.; Rizzo, B.; Caliceti, C.; Landi, L.; Hrelia, S.; Prata, C. Specific aquaporins facilitate NOX-produced hydrogen peroxide transport through plasma membrane in leukaemia cells. Biochim. Biophys. Acta 2014, 1843, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Moniaga, C.S.; Nielsen, S.; Hara-Chikuma, M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016, 471, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Hara-Chikuma, M. Aquaporin-3 controls breast cancer cell migration by regulating hydrogen peroxide transport and its downstream cell signaling. Mol. Cell. Biol. 2016, 36, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Oberg, F.; Sjohamn, J.; Hedfalk, K.; Bill, R.M.; Conner, A.C.; Conner, M.T.; Tornroth-Horsefield, S. Plasma membrane abundance of human aquaporin 5 is dynamically regulated by multiple pathways. PLoS ONE 2015, 10, e0143027. [Google Scholar] [CrossRef] [PubMed]
- Soveral, G.; Madeira, A.; Loureiro-Dias, M.C.; Moura, T.F. Membrane tension regulates water transport in yeast. Biochim. Biophys. Acta 2008, 1778, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Ozu, M.; Dorr, R.A.; Gutierrez, F.; Politi, M.T.; Toriano, R. Human AQP1 is a constitutively open channel that closes by a membrane-tension-mediated mechanism. Biophys. J. 2013, 104, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Leitao, L.; Prista, C.; Loureiro-Dias, M.C.; Moura, T.F.; Soveral, G. The grapevine tonoplast aquaporin TIP2;1 is a pressure gated water channel. Biochem. Biophys. Res. Commun. 2014, 450, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Tornroth-Horsefield, S.; Hedfalk, K.; Fischer, G.; Lindkvist-Petersson, K.; Neutze, R. Structural insights into eukaryotic aquaporin regulation. FEBS Lett. 2010, 584, 2580–2588. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Kosinska-Eriksson, U.; Aponte-Santamaria, C.; Palmgren, M.; Geijer, C.; Hedfalk, K.; Hohmann, S.; de Groot, B.L.; Neutze, R.; Lindkvist-Petersson, K. Crystal structure of a yeast aquaporin at 1.15 angstrom reveals a novel gating mechanism. PLoS Biol. 2009, 7, e1000130. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, N.; Hagstrom, J.; Bill, R.M.; Hohmann, S. Expression of heterologous aquaporins for functional analysis in Saccharomyces cerevisiae. Curr. Genet. 2006, 50, 247–255. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.; Martins, A.P.; Mosca, A.F.; Wijma, H.J.; Prista, C.; Soveral, G.; Casini, A. Exploring the gating mechanisms of aquaporin-3: New clues for the design of inhibitors? Mol. Biosyst. 2016, 12, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Leitao, L.; Prista, C.; Moura, T.F.; Loureiro-Dias, M.C.; Soveral, G. Grapevine aquaporins: Gating of a tonoplast intrinsic protein (TIP2;1) by cytosolic ph. PLoS ONE 2012, 7, e33219. [Google Scholar] [CrossRef] [PubMed]
- Sabir, F.; Leandro, M.J.; Martins, A.P.; Loureiro-Dias, M.C.; Moura, T.F.; Soveral, G.; Prista, C. Exploring three pips and three tips of grapevine for transport of water and atypical substrates through heterologous expression in aqy-null yeast. PLoS ONE 2014, 9, e102087. [Google Scholar] [CrossRef] [PubMed]
- Yasui, M.; Hazama, A.; Kwon, T.H.; Nielsen, S.; Guggino, W.B.; Agre, P. Rapid gating and anion permeability of an intracellular aquaporin. Nature 1999, 402, 184–187. [Google Scholar] [PubMed]
- Zelenina, M.; Bondar, A.A.; Zelenin, S.; Aperia, A. Nickel and extracellular acidification inhibit the water permeability of human aquaporin-3 in lung epithelial cells. J. Biol. Chem. 2003, 278, 30037–30043. [Google Scholar] [CrossRef] [PubMed]
- Nemeth-Cahalan, K.L.; Hall, J.E. Ph and calcium regulate the water permeability of aquaporin 0. J. Biol. Chem. 2000, 275, 6777–6782. [Google Scholar] [CrossRef] [PubMed]
- Janosi, L.; Ceccarelli, M. The gating mechanism of the human aquaporin 5 revealed by molecular dynamics simulations. PLoS ONE 2013, 8, e59897. [Google Scholar] [CrossRef] [PubMed]
- Alberga, D.; Nicolotti, O.; Lattanzi, G.; Nicchia, G.P.; Frigeri, A.; Pisani, F.; Benfenati, V.; Mangiatordi, G.F. A new gating site in human aquaporin-4: Insights from molecular dynamics simulations. BBA-Biomembranes 2014, 1838, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Kaptan, S.; Assentoft, M.; Schneider, H.P.; Fenton, R.A.; Deitmer, J.W.; MacAulay, N.; de Groot, B.L. H95 is a pH-dependent gate in aquaporin 4. Structure 2015, 23, 2309–2318. [Google Scholar] [CrossRef] [PubMed]
- Yukutake, Y.; Yasui, M. Regulation of water permeability through aquaporin-4. Neuroscience 2010, 168, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped blast and PSI-blast: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Orij, R.; Postmus, J.; Ter Beek, A.; Brul, S.; Smits, G.J. In vivo measurement of cytosolic and mitochondrial pH using a pH-sensitive GFP derivative in Saccharomyces cerevisiae reveals a relation between intracellular pH and growth. Microbiology 2009, 155, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Kosugi-Tanaka, C.; Li, X.; Yao, C.; Akamatsu, T.; Kanamori, N.; Hosoi, K. Protein kinase a-regulated membrane trafficking of a green fluorescent protein-aquaporin 5 chimera in MDCK cells. Biochim. Biophys Acta 2006, 1763, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Kawedia, J.D.; Menon, A.G. Cyclic AMP regulates aquaporin 5 expression at both transcriptional and post-transcriptional levels through a protein kinase a pathway. J. Biol. Chem. 2003, 278, 32173–32180. [Google Scholar] [CrossRef] [PubMed]
- Sidhaye, V.; Hoffert, J.D.; King, L.S. Camp has distinct acute and chronic effects on aquaporin-5 in lung epithelial cells. J. Biol. Chem. 2005, 280, 3590–3596. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Lee, J.; Chae, Y.K.; Kim, M.S.; Baek, J.H.; Park, J.C.; Park, M.J.; Smith, I.M.; Trink, B.; Ratovitski, E.; et al. Overexpression of AQP5, a putative oncogene, promotes cell growth and transformation. Cancer Lett. 2008, 264, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, R.; Konijn, T.M. Cyclic 3’,5’-AMP in Saccharomyces carlsbergensis under various conditions of catabolite repression. FEBS Lett. 1971, 13, 184–186. [Google Scholar] [CrossRef]
- Mbonyi, K.; van Aelst, L.; Arguelles, J.C.; Jans, A.W.; Thevelein, J.M. Glucose-induced hyperaccumulation of cyclic AMP and defective glucose repression in yeast strains with reduced activity of cyclic AMP-dependent protein kinase. Mol. Cell. Biol. 1990, 10, 4518–4523. [Google Scholar] [CrossRef] [PubMed]
- Thevelein, J.M.; de Winde, J.H. Novel sensing mechanisms and targets for the CAMP-protein kinase a pathway in the yeast Saccharomyces cerevisiae. Mol. Microbial. 1999, 33, 904–918. [Google Scholar] [CrossRef]
- Johnston, M.; Kim, J.H. Glucose as a hormone: Receptor-mediated glucose sensing in the yeast Saccharomyces cerevisiae. Biochem. Soc. Trans. 2005, 33, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Van der Plaat, J.B. Cyclic 3’,5’-adenosine monophosphate stimulates trehalose degradation in baker’s yeast. Biochem. Biophys. Res. Commun. 1974, 56, 580–587. [Google Scholar] [CrossRef]
- Rolland, F.; Wanke, V.; Cauwenberg, L.; Ma, P.; Boles, E.; Vanoni, M.; de Winde, J.H.; Thevelein, J.M.; Winderickx, J. The role of hexose transport and phosphorylation in CAMP signalling in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2001, 1, 33–45. [Google Scholar] [PubMed]
- Horsefield, R.; Norden, K.; Fellert, M.; Backmark, A.; Tornroth-Horsefield, S.; Terwisscha van Scheltinga, A.C.; Kvassman, J.; Kjellbom, P.; Johanson, U.; Neutze, R. High-resolution X-ray structure of human aquaporin 5. Proc. Natl. Acad. Sci. USA 2008, 105, 13327–13332. [Google Scholar] [CrossRef] [PubMed]
- Eto, K.; Noda, Y.; Horikawa, S.; Uchida, S.; Sasaki, S. Phosphorylation of aquaporin-2 regulates its water permeability. J. Biol. Chem. 2010, 285, 40777–40784. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Fushimi, K.; Terada, Y.; Bai, L.; Marumo, F.; Sasaki, S. cAMP-dependent phosphorylation stimulates water permeability of aquaporin-collecting duct water channel protein expressed in xenopus oocytes. J. Biol. Chem. 1995, 270, 10384–10387. [Google Scholar] [PubMed]
- Choi, H.J.; Jung, H.J.; Kwon, T.H. Extracellular pH affects phosphorylation and intracellular trafficking of AQP2 in inner medullary collecting duct cells. Am. J. Physiol. Ren. Physiol. 2015, 308, F737–F748. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.R.; Marinho, H.S.; Cyrne, L.; Antunes, F. Decrease of H2O2 plasma membrane permeability during adaptation to H2O2 in Saccharomyces cerevisiae. J. Biol. Chem. 2004, 279, 6501–6506. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, F.; Mandol, L. Studies of pH of sweat produced by different forms of stimulation. J. Investig. Dermatol. 1955, 24, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.; Pietrement, C.; Brown, D.; Breton, S. Segmental and cellular expression of aquaporins in the male excurrent duct. Biochim. Biophys. Acta 2006, 1758, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Lin, M.; Yang, J.H.; Chen, J.F.; Tu, Y.R. Overexpression of AQP5 was detected in axillary sweat glands of primary focal hyperhidrosis patients. Dermatology 2016, 232, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Parvin, M.N.; Tsumura, K.; Akamatsu, T.; Kanamori, N.; Hosoi, K. Expression and localization of AQP5 in the stomach and duodenum of the rat. Biochim. Biophys. Acta 2002, 1542, 116–124. [Google Scholar] [CrossRef]
- Yi, F.; Khan, M.; Gao, H.; Hao, F.; Sun, M.; Zhong, L.; Lu, C.; Feng, X.; Ma, T. Increased differentiation capacity of bone marrow-derived mesenchymal stem cells in aquaporin-5 deficiency. Stem Cells Dev. 2012, 21, 2495–2507. [Google Scholar] [CrossRef] [PubMed]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.; Moradas-Ferreira, P. Oxidative stress and signal transduction in Saccharomyces cerevisiae: Insights into ageing, apoptosis and diseases. Mol. Asp. Med. 2001, 22, 217–246. [Google Scholar] [CrossRef]
- Farrugia, G.; Balzan, R. Oxidative stress and programmed cell death in yeast. Front. Oncol. 2012, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Ikner, A.; Shiozaki, K. Yeast signaling pathways in the oxidative stress response. Mutat. Res. 2005, 569, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Vollgraf, U.; Wegner, M.; Richter-Landsberg, C. Activation of AP-1 and nuclear factor-κB transcription factors is involved in hydrogen peroxide-induced apoptotic cell death of oligodendrocytes. J. Neurochem. 1999, 73, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Krane, C.M.; Towne, J.E.; Menon, A.G. Cloning and characterization of murine AQP5: Evidence for a conserved aquaporin gene cluster. Mamm. Genome 1999, 10, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Guldener, U.; Heck, S.; Fielder, T.; Beinhauer, J.; Hegemann, J.H. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996, 24, 2519–2524. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Techniques for Transformation of Escherichia coli; IRL Press: Oxford, UK, 1985. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor: New York, NY, USA, 1989. [Google Scholar]
- Pronk, J.T. Auxotrophic yeast strains in fundamental and applied research. Appl. Environ. Microbiol. 2002, 68, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Geitz, R.D.; Schiestl, R.H. Transforming yeast with DNA. Methods Mol. Cell. Biol. 1995, 5, 255–269. [Google Scholar]
- Joshi, S.D.; Davidson, L.A. Live-cell imaging and quantitative analysis of embryonic epithelial cells in Xenopus laevis. J. Vis. Exp. 2010. [Google Scholar] [CrossRef] [PubMed]
- Soveral, G.; Madeira, A.; Loureiro-Dias, M.C.; Moura, T.F. Water transport in intact yeast cells as assessed by fluorescence self-quenching. Appl. Environ. Microbiol. 2007, 73, 2341–2343. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, C.; Mósca, A.F.; Martins, A.P.; Nobre, T.; Prista, C.; Antunes, F.; Cipak Gasparovic, A.; Soveral, G. Rat Aquaporin-5 Is pH-Gated Induced by Phosphorylation and Is Implicated in Oxidative Stress. Int. J. Mol. Sci. 2016, 17, 2090. https://doi.org/10.3390/ijms17122090
Rodrigues C, Mósca AF, Martins AP, Nobre T, Prista C, Antunes F, Cipak Gasparovic A, Soveral G. Rat Aquaporin-5 Is pH-Gated Induced by Phosphorylation and Is Implicated in Oxidative Stress. International Journal of Molecular Sciences. 2016; 17(12):2090. https://doi.org/10.3390/ijms17122090
Chicago/Turabian StyleRodrigues, Claudia, Andreia Filipa Mósca, Ana Paula Martins, Tatiana Nobre, Catarina Prista, Fernando Antunes, Ana Cipak Gasparovic, and Graça Soveral. 2016. "Rat Aquaporin-5 Is pH-Gated Induced by Phosphorylation and Is Implicated in Oxidative Stress" International Journal of Molecular Sciences 17, no. 12: 2090. https://doi.org/10.3390/ijms17122090
APA StyleRodrigues, C., Mósca, A. F., Martins, A. P., Nobre, T., Prista, C., Antunes, F., Cipak Gasparovic, A., & Soveral, G. (2016). Rat Aquaporin-5 Is pH-Gated Induced by Phosphorylation and Is Implicated in Oxidative Stress. International Journal of Molecular Sciences, 17(12), 2090. https://doi.org/10.3390/ijms17122090