Nocturnal Pruritus: The Battle for a Peaceful Night’s Sleep
Abstract
:1. Introduction
2. The Importance of Sleep
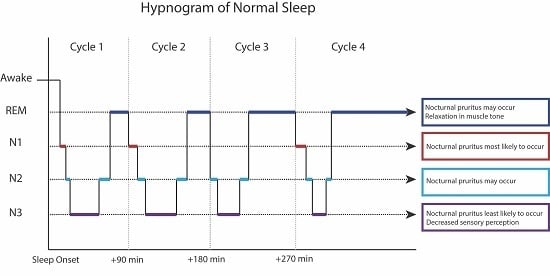
3. Stages of Sleep
4. Physiological Mechanisms for Nocturnal Pruritus
5. Conditions that Cause Nocturnal Itch
6. Sleep Monitoring
7. Treatments for Nocturnal Pruritus
7.1. Antihistamines
7.2. Antidepressants
7.3. Gabaergics
7.4. κ-Opioid Agonists
7.5. Benzodiazepines
7.6. Hormones
7.7. Psychological Interventions
8. Conclusions
Conflicts of Interest
References
- Stander, S.; Weisshaar, E.; Mettang, T.; Szepietowski, J.C.; Carstens, E.; Ikoma, A.; Bergasa, N.V.; Gieler, U.; Misery, L.; Wallengren, J.; et al. Clinical classification of itch: A position paper of the international forum for the study of itch. Acta Derm. Venereol. 2007, 87, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Goon, A.; Wee, J.; Chan, Y.H.; Goh, C.L. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br. J. Dermatol. 2000, 143, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Ansari, N.; Goon, A.; Chan, Y.H.; Goh, C.L. Clinical characteristics of pruritus in chronic idiopathic urticaria. Br. J. Dermatol. 2002, 147, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Goon, A.T.; Wee, J.; Chan, Y.H.; Zucker, I.; Goh, C.L. Itch characteristics in Chinese patients with atopic dermatitis using a new questionnaire for the assessment of pruritus. Int. J. Dermatol. 2002, 41, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Pisoni, R.L.; Wikström, B.; Elder, S.J.; Akizawa, T.; Asano, Y.; Keen, M.L.; Saran, R.; Mendelssohn, D.C.; Young, E.W.; Port, F.K. Pruritus in haemodialysis patients: International results from the dialysis outcomes and practice patterns study (DOPPS). Nephrol. Dial. Transplant. 2006, 21, 3495–3505. [Google Scholar] [CrossRef] [PubMed]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef]
- Ruiz, F.S.; Andersen, M.L.; Martins, R.C.; Zager, A.; Lopes, J.D.; Tufik, S. Immune alterations after selective rapid eye movement or total sleep deprivation in healthy male volunteers. Innate Immun. 2012, 18, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Porkka-Heiskanen, T. Sleep homeostasis. Curr. Opin. Neurobiol. 2013, 23, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Imeri, L.; Opp, M.R. How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 2009, 10, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R. Why sleep is important for health: A psychoneuroimmunology perspective. Annu. Rev. Psychol. 2015, 66, 143–172. [Google Scholar] [CrossRef] [PubMed]
- Benca, R. Sleep Disorders: The Clinician’s Guide to Diagnosis and Management; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Dinges, D.F.; Pack, F.; Williams, K.; Gillen, K.A.; Powell, J.W.; Ott, G.E.; Aptowicz, C.; Pack, A.I. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep 1997, 20, 267–277. [Google Scholar] [PubMed]
- Daley, M.; Morin, C.M.; LeBlanc, M.; Gregoire, J.P.; Savard, J.; Baillargeon, L. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2009, 10, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Leproult, R.; van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Tochikubo, O.; Ikeda, A.; Miyajima, E.; Ishii, M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension 1996, 27, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended amount of sleep for a healthy adult: A joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.; Howard, R.; Rossor, M.; Shorvon, S. Neurology: A Queen Square Textbook; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Chokroverty, S. Overview of sleep & sleep disorders. Indian J. Med. Res. 2010, 131, 126–140. [Google Scholar] [PubMed]
- Aoki, T.; Kushimoto, H.; Hishikawa, Y.; Savin, J.A. Nocturnal scratching and its relationship to the disturbed sleep of itchy subjects. Clin. Exp. Dermatol. 1991, 16, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Savin, J.A.; Paterson, W.D.; Oswald, I.; Adam, K. Further studies of scratching during sleep. Br. J. Dermatol. 1975, 93, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M.; Vignale, R.; Monti, D. Sleep and nighttime pruritus in children with atopic dermatitis. Sleep 1989, 12, 309–314. [Google Scholar] [PubMed]
- Koca, R.; Altin, R.; Konuk, N.; Altinyazar, H.C.; Kart, L. Sleep disturbance in patients with lichen simplex chronicus and its relationship to nocturnal scratching: A case control study. South Med. J. 2006, 99, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Chou, Y.T.; Lee, J.H.; Lee, P.L.; Dai, Y.S.; Sun, C.; Lin, Y.T.; Wang, L.C.; Yu, H.H.; Yang, Y.H.; et al. Atopic Dermatitis, melatonin and sleep disturbance. Pediatrics 2014, 134, e397–e405. [Google Scholar] [CrossRef] [PubMed]
- Reuveni, H.; Chapnick, G.; Tal, A.; Tarasiuk, A. Sleep fragmentation in children with atopic dermatitis. Arch. Pediatr. Adolesc. Med. 1999, 153, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, A.; Steinhoff, M.; Ständer, S.; Yosipovitch, G.; Schmelz, M. The neurobiology of itch. Nat. Rev. Neurosci. 2006, 7, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Dhand, A.; Aminoff, M.J. The neurology of itch. Brain 2014, 137, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Nigam, G.; Riaz, M.; Hershner, S.D.; Goldstein, C.A.; Chervin, R.D. Sleep Related Scratching: A Distinct Parasomnia? J. Clin. Sleep Med. 2016, 12, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Thornburn, P.T.; Riha, R.L. Skin disorder and sleep in adults: Where is the evidence? Sleep Med. Rev. 2010, 14, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.A.; Simpson, F.C.; Gupta, A.K. Psoriasis and sleep disorders: A systematic review. Sleep Med. Rev. 2016, 29, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Xiong, G.L.; Haus, E.; Sackett-Lundeen, L.; Ashkenazi, I.; Maibach, H.I. Time-dependent variations of the skin barrier function in humans: Transepidermal water loss, stratum corneum hydration, skin surface pH, and skin temperature. J. Investig. Dermatol 1998, 110, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chuang, H.Y.; Shih, C.C.; Jong, S.B.; Chang, C.H.; Yu, H.S. Transepidermal water loss, serum IgE and β endorphin as important and independent biological markers for development of itch intesnity in atopic dermatitis. Br. J. Dermatol. 2006, 154, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Reinberg, A.E.; Martin, R.J.; Haus, E. Clinical chronobiology and chronotherapeutics with applications to asthma. Chronobiol. Int. 1999, 16, 539–563. [Google Scholar] [CrossRef] [PubMed]
- Honma, Y.; Arai, I.; Hashimoto, Y.; Futaki, N.; Sugimoto, M.; Tanaka, M.; Nakaike, S. Prostaglandin D2 and prostaglandin E3 accelerate the recovery of cutaneous barrier disruption induced by mechanical scratching in mice. Eur. J. Pharmacol. 2005, 518, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Ishiuji, Y.; Yosipovitch, G. Nocturnal itch: Why do we itch at night? Acta Derm. Venereol. 2007, 87, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Leslie, T.A.; Greaves, M.W.; Yosipovitch, G. Current topical and systemic therapies for itch. Handb. Exp. Pharmacol. 2015, 226, 337–356. [Google Scholar] [PubMed]
- Sugimoto, M.; Arai, I.; Futaki, N.; Hashimoto, Y.; Honma, Y.; Nakaike, S. COX-1 inhibition enhances scratching behavior in NC/Nga mice with atopic dermatitis. Exp. Dermatol. 2006, 15, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Stull, C.; Lavery, M.J.; Yosipovitch, G. Advances in therapeutic strategies for the treatment of pruritus. Expert Opin. Pharmacother. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Rovelli, F.; Brivio, F.; Brivio, O.; Fumagalli, L. Circadian secretions of IL-2, IL-12, IL-6 and IL-10 in relation to the light/dark rhythm of the pineal hormone melatonin in healthy humans. Nat. Immun. 1998, 16, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Bienenstock, J.; Schmelz, M.; Maurer, M.; Wei, E.; Bíró, T. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J. Investig. Dermatol. 2006, 126, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Papoiu, A.D.P.; Wang, H.; Nattkemper, L.; Tey, H.L.; Ishiuji, Y.; Chan, Y.H.; Schmelz, M.; Yosipovitch, G. A study of serum concentrations and dermal levels of NGF in atopic dermatitis and healthy subjects. Neuropeptides 2011, 45, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, W.; Birau, N.; Hornstein, O.P.; Heubeck, B.; Schönberger, A.; Meyer, C.; Gottschalk, J. Alterations of melatonin secretion in atopic eczema. Acta Derm. Venereol. 1988, 68, 224–229. [Google Scholar] [PubMed]
- Gelder, M.; Mayou, R.; Geddes, J. Mood disorders. In Psychiatry, 3rd ed.; Oxford University Press: Oxford, UK, 2005; pp. 97–118. [Google Scholar]
- Camfferman, D.; Kennedy, J.D.; Gold, M. Eczema and sleep and its relationship to daytime functioning in children. Sleep Med. Rev. 2010, 14, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Sack, R.; Hanifin, J. Scratching below the surface of sleep and itch. Sleep Med. Rev. 2010, 14, 349–350. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Garg, N.K.; Paller, A.S.; Fishbein, A.B.; Zee, P.C. Sleep disturbances in adults with eczema are associated with impaired overall health: A US population-based study. J. Investig. Dermatol. 2015, 135, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.E.; Currie, G.P.; Koudelka, C.W.; Simpson, E.L. Eczema prevalence in the United States: Data from the 2003 national survey of children’s health. J. Investig. Dermatol. 2011, 131, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mollanazar, N.K.; Smith, P.K.; Yosipovitch, G. Mediators of chronic pruritus in atopic dermatitis: Getting the itch out? Clin. Rev. Allergy Immunol. 2015, 1, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, E.; Seidman, D.S.; Laor, A.; Shapiro, Y.; Epstein, Y. Are psoriatic patients at risk of heat intolerance? Br. J. Dermatol. 1991, 124, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Rider, S.D., Jr.; Morgan, M.S.; Arlian, L.G. Draft genome of the scabies mite. Parsit. Vectors 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Lavery, M.J.; Parish, L.C. Bed bugs revisited. Skinmed 2011, 9, 6–8. [Google Scholar] [PubMed]
- Rosenfeld, J.A.; Reeves, D.; Brugler, M.R.; Narechania, A.; Simon, S.; Durrett, R.; Foox, J.; Shianna, K.; Schatz, M.C.; Gandara, J.; et al. Genome assemnly and geospatial phylogenomics of the bed bug Cimex lectularius. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Rodriguez, R.; Stull, C.; Yosipovitch, G. Chronic pruritus in the elderly: Pathophysiology, diagnosis and management. Drugs Aging 2015, 32, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Mazeh, D.; Melamed, Y.; Cholostoy, A.; Aharonovitzch, V.; Weizman, A.; Yosipovitch, G. Itching in the psychiatric ward. Acta Derm. Venereol. 2008, 88, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Bender, B.G.; Ballard, R.; Canono, B.; Murphy, J.R.; Leung, D.Y. Disease severity, scratching, and sleep quality in patients with atopic dermatitis. J. Am. Acad. Dermatol. 2008, 58, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Bender, B.G.; Leung, S.B.; Leung, D.Y. Actigraphy assessment of sleep disturbance in patients with atopic dermatitis: An objective life quality measure. J. Allergy Clin. Immunol. 2003, 111, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Bringhurst, C.; Waterston, K.; Schofield, O.; Benjamin, K.; Rees, J.L. Measurement of itch using actigraphy in pediatric and adult populations. J. Am. Acad. Dermatol. 2004, 51, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Ebata, T.; Iwasaki, S.; Kamide, R.; Niimura, M. Use of a wrist activity monitor for the measurement of nocturnal scratching in patients with atopic dermatitis. Br. J. Dermatol. 2001, 144, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, K.; Waterston, K.; Russell, M.; Schofield, O.; Diffey, B.; Rees, J.L. The development of an objective method for measuring scratch in children with atopic dermatitis suitable for clinical use. J. Am. Acad. Dermatol. 2004, 50, 33–40. [Google Scholar] [CrossRef]
- Murray, C.S.; Rees, J.L. Are subjective accounts of itch to be relied on? The lack of relation between visual analogue itch scores and actigraphic measures of scratch. Acta Derm. Venereol. 2011, 91, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Yosipovitch, G. Therapy of pruritus. Expert Opin. Pharmacother. 2010, 11, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Motomura, E.; Noro, Y.; Umeda, K.; Morikawa, T.; Umeda-Togami, K.; Omoto, Y.; Isoda, K.; Kondo, M.; Tsuda, K.; et al. Olopatadine, a non-sedating H1 antihistamine, decreases the nocturnal scratching without affecting sleep quality in atopic dermatitis. Exp. Dermatol. 2015, 24, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Hundley, J.L.; Yosipovitch, G. Mirtazapine for reducing nocturnal itch in patients with chronic pruritus: A pilot study. J. Am. Acad. Dermatol. 2004, 50, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P.; Frandsen, J.L.; Walsh, D.; Andresen, S.; Taylor, S. Mirtazapine for pruritus. J. Pain Symptom Manag. 2003, 25, 288–291. [Google Scholar] [CrossRef]
- Puzantian, T. Mirtazapine, an antidepressant. Am. J. Health Syst. Pharm. 1998, 55, 44–49. [Google Scholar] [PubMed]
- Yosipovitch, G.; Samuel, L.S. Neuropathic and psychogenic itch. Dermatol. Ther. 2008, 21, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Dawn, A.G.; Yosipovitch, G. Butorphanol for treatment of intractable pruritus. J. Am. Acad. Dermatol. 2006, 54, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Roux, F.J.; Kryger, M.H. Medication effects on sleep. Clin. Chest Med. 2010, 31, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Ebata, T.; Izumi, H.; Aizawa, H.; Kamide, R.; Niimura, M. Effects of nitrazepam on nocturnal scratching in adults with atopic dermatitis: A double-blind placebo-controlled crossover study. Br. J. Dermatol. 1998, 138, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Asnis, G.M.; Thomas, M.; Henderson, M.A. Pharmacotherapy treatment options for insomnia: A primer for clinicians. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Lin, M.H.; Lee, J.H.; Lee, P.L.; Dai, Y.S.; Chu, K.H.; Sun, C.; Lin, Y.T.; Wang, L.C.; Yu, H.H.; et al. Melatonin supplementation for children with atopic dermatitis and sleep disturbance: A randomized clinical trial. JAMA Pediatr. 2016, 170, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Schut, C.; Mollanazar, N.K.; Kupfer, J.; Gieler, U.; Yosipovitch, G. Psychological interventions in the treatment of chronic itch. Acta Derm. Venereol. 2016, 96, 157–161. [Google Scholar] [CrossRef] [PubMed]

| Stage of Sleep | Electroencephalogram (EEG) Characteristics and Associated Sleep Stage Features |
|---|---|
| Wakefulness | Mainly alpha (8–13 Hz) and beta activity (>13 Hz) on EEG |
| NREM Sleep | |
| N1 (formerly Stage 1) (10% of sleep time) | Slow theta waves (4–7 Hz) emerge on EEG |
| Rolling eye movements | |
| Lightest stage of sleep | |
| N2 (formerly Stage 2) (50% of sleep time) | Background theta wave, less than 20% delta waves. Sleep spindles (central 14–16 Hz rhythms) and K complexes (0.5–1 s high amplitude central sharp waves) on EEG. |
| Eye movements usually cease | |
| N3 (formerly Stages 3 + 4) (20% of sleep time) | High amplitude delta waves (<4 Hz) on EEG Also termed slow wave sleep |
| Eye movements are slow or absent | |
| Deepest stage of sleep | |
| REM Sleep (20% of sleep time) | Low voltage, mixed frequency background on EEG |
| Saccadic eye movements | |
| 80% of dreams occur in this stage. They may be recalled easily especially if immediate awakening after dream onset | |
| Category | Disease |
|---|---|
| Dermatological | Atopic dermatitis |
| Psoriasis | |
| Chronic idiopathic urticaria | |
| Infestations (scabies, bed bugs, pediculosis, pinworms) | |
| Lichen planus | |
| Lichen simplex chronicus | |
| Prurigo nodularis | |
| Non-Dermatological | Liver disease |
| Chronic kidney disease | |
| Hematopoietic disorders | |
| Neurological (e.g., brachioradial pruritus) | |
| Psychological (delusional ideations, depression, schizophrenia, stress) | |
| Substance abuse | |
| Advanced age (senile) pruritus | |
| Restless legs syndrome |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavery, M.J.; Stull, C.; Kinney, M.O.; Yosipovitch, G. Nocturnal Pruritus: The Battle for a Peaceful Night’s Sleep. Int. J. Mol. Sci. 2016, 17, 425. https://doi.org/10.3390/ijms17030425
Lavery MJ, Stull C, Kinney MO, Yosipovitch G. Nocturnal Pruritus: The Battle for a Peaceful Night’s Sleep. International Journal of Molecular Sciences. 2016; 17(3):425. https://doi.org/10.3390/ijms17030425
Chicago/Turabian StyleLavery, Michael Joseph, Carolyn Stull, Michael Owen Kinney, and Gil Yosipovitch. 2016. "Nocturnal Pruritus: The Battle for a Peaceful Night’s Sleep" International Journal of Molecular Sciences 17, no. 3: 425. https://doi.org/10.3390/ijms17030425
APA StyleLavery, M. J., Stull, C., Kinney, M. O., & Yosipovitch, G. (2016). Nocturnal Pruritus: The Battle for a Peaceful Night’s Sleep. International Journal of Molecular Sciences, 17(3), 425. https://doi.org/10.3390/ijms17030425