MMP9 Rs3918242 Polymorphism Affects Tachycardia-Induced MMP9 Expression in Cultured Atrial-Derived Myocytes but Is Not a Risk Factor for Atrial Fibrillation among the Taiwanese
Abstract
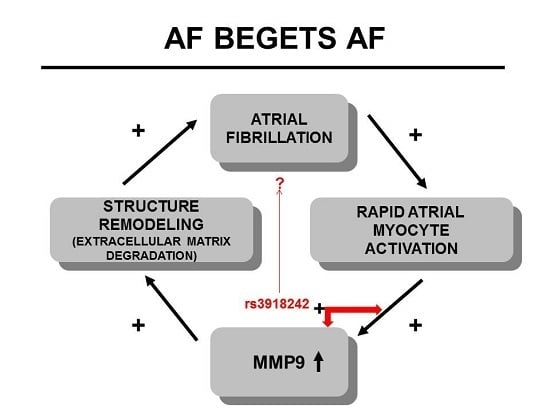
:1. Introduction
2. Results
2.1. Rapid Pacing Induces MMP9 Expression and Secretion of HL-1 Atrial Myocytes in a Time- and Dose-Dependent Manner
2.2. Effect of the Rs3918242 on the MMP9 Transcriptional Activity in Rapidly Stimulated HL-1 Myocytes
2.3. SNP rs3918242 in the MMP9 Promoter Is Not Associated with AF
2.4. Lack of Association of SNP rs3918242 with MMP9 Expression in Atrial Tissues
3. Discussion
4. Experimental Section
4.1. Ethics Statement
4.2. Study Population
4.3. Clinical Assessment
4.4. Genomic DNA Extraction and Genotyping of the Rs3918242
4.5. Human Samples
4.6. Cell Culture and Tachypacing
4.7. Immunoblotting
4.8. MMP9 Gelatin Zymography
4.9. MMP9 Promoter Activity Assay
4.10. Immunohistochemical Analysis
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Wolf, P.A.; Benjamin, E.J.; Levy, D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am. J. Cardiol. 1998, 82, 2N–9N. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Vasan, R.S.; Leip, E.P.; Wolf, P.A.; D’Agostino, R.B.; Murabito, J.M.; Kannel, W.B.; Benjamin, E.J. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The framingham heart study. Circulation 2003, 107, 2920–2925. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of atrial fibrillation on the risk of death: The framingham heart study. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Allessie, M.; Ausma, J.; Schotten, U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc. Res. 2002, 54, 230–246. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the american college of cardiology/american heart association task force on practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 2014, 64, e1–e76. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Burstein, B.; Dobrev, D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ. Arrhythm. Electrophysiol. 2008, 1, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Polyakova, V.; Miyagawa, S.; Szalay, Z.; Risteli, J.; Kostin, S. Atrial extracellular matrix remodelling in patients with atrial fibrillation. J. Cell. Mol. Med. 2008, 12, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Creemers, E.E.; Cleutjens, J.P.; Smits, J.F.; Daemen, M.J. Matrix metalloproteinase inhibition after myocardial infarction: A new approach to prevent heart failure? Circ. Res. 2001, 89, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G. Matrix metalloproteinases: Regulation and dysregulation in the failing heart. Circ. Res. 2002, 90, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Niida, S.; Dote, K.; Takenaka, S.; Hirao, H.; Miura, F.; Ishida, M.; Shingu, T.; Sueda, T.; Yoshizumi, M.; et al. Matrix metalloproteinase-9 contributes to human atrial remodeling during atrial fibrillation. J. Am. Coll. Cardiol. 2004, 43, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.K.; Lucero, J.; Lucas, S.; Madden, M.C.; McDonald, J.D.; Seagrave, J.C.; Knuckles, T.L.; Campen, M.J. Vehicular emissions induce vascular MMP-9 expression and activity associated with endothelin-1-mediated pathways. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Lopez, F.L.; MacLehose, R.F.; Eckfeldt, J.H.; Couper, D.; Leiendecker-Foster, C.; Hoogeveen, R.C.; Chen, L.Y.; Soliman, E.Z.; Agarwal, S.K.; et al. Novel association between plasma matrix metalloproteinase-9 and risk of incident atrial fibrillation in a case-cohort study: The atherosclerosis risk in communities study. PLoS ONE 2013, 8, e59052. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, G.; Xie, B.; Babu, K.; Huang, C. Changes in matrix metalloproteinase-9 levels during progression of atrial fibrillation. J. Int. Med. Res. 2014, 42, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, A.S.; Tsiodras, S.; Rigopoulos, A.G.; Sakadakis, E.A.; Triantafyllis, A.; Kremastinos, D.T.; Rizos, I. Novel association patterns of cardiac remodeling markers in patients with essential hypertension and atrial fibrillation. BMC Cardiovasc. Disord. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hsu, L.A.; Teng, M.S.; Lin, J.F.; Chang, H.H.; Chang, P.Y.; Hu, C.F.; Ko, Y.L. Association of matrix metalloproteinase 9 genotypes and cardiovascular disease risk factors with serum matrix metalloproteinase 9 concentrations in taiwanese individuals. Clin. Chem. Lab. Med. 2010, 48, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ye, S.; Herrmann, S.M.; Eriksson, P.; de Maat, M.; Evans, A.; Arveiler, D.; Luc, G.; Cambien, F.; Hamsten, A.; et al. Functional polymorphism in the regulatory region of gelatinase b gene in relation to severity of coronary atherosclerosis. Circulation 1999, 99, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Burstein, B.; Qi, X.Y.; Yeh, Y.H.; Calderone, A.; Nattel, S. Atrial cardiomyocyte tachycardia alters cardiac fibroblast function: A novel consideration in atrial remodeling. Cardiovasc. Res. 2007, 76, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shen, W.; Rottman, J.N.; Wikswo, J.P.; Murray, K.T. Rapid stimulation causes electrical remodeling in cultured atrial myocytes. J. Mol. Cell. Cardiol. 2005, 38, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Mace, L.C.; Yermalitskaya, L.V.; Yi, Y.; Yang, Z.; Morgan, A.M.; Murray, K.T. Transcriptional remodeling of rapidly stimulated HL-1 atrial myocytes exhibits concordance with human atrial fibrillation. J. Mol. Cell. Cardiol. 2009, 47, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.H.; Kuo, C.T.; Chan, T.H.; Chang, G.J.; Qi, X.Y.; Tsai, F.; Nattel, S.; Chen, W.J. Transforming growth factor-β and oxidative stress mediate tachycardia-induced cellular remodelling in cultured atrial-derived myocytes. Cardiovasc. Res. 2011, 91, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Hoit, B.D.; Takeishi, Y.; Cox, M.J.; Gabel, M.; Kirkpatrick, D.; Walsh, R.A.; Tyagi, S.C. Remodeling of the left atrium in pacing-induced atrial cardiomyopathy. Mol. Cell. Biochem. 2002, 238, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Huang, S.K.; Lin, J.L.; Lai, L.P.; Lai, S.C.; Liu, C.W.; Chen, W.C.; Wen, C.H.; Lin, C.S. Upregulation of matrix metalloproteinase-9 and tissue inhibitors of metalloproteinases in rapid atrial pacing-induced atrial fibrillation. J. Mol. Cell. Cardiol. 2008, 45, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Ellinor, P.T.; Lunetta, K.L.; Albert, C.M.; Glazer, N.L.; Ritchie, M.D.; Smith, A.V.; Arking, D.E.; Muller-Nurasyid, M.; Krijthe, B.P.; Lubitz, S.A.; et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat. Genet. 2012, 44, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, D.F.; Holm, H.; Gretarsdottir, S.; Thorleifsson, G.; Walters, G.B.; Thorgeirsson, G.; Gulcher, J.; Mathiesen, E.B.; Njolstad, I.; Nyrnes, A.; et al. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat. Genet. 2009, 41, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Rice, K.M.; Arking, D.E.; Pfeufer, A.; van Noord, C.; Smith, A.V.; Schnabel, R.B.; Bis, J.C.; Boerwinkle, E.; Sinner, M.F.; et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of european ancestry. Nat. Genet. 2009, 41, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, D.F.; Arnar, D.O.; Helgadottir, A.; Gretarsdottir, S.; Holm, H.; Sigurdsson, A.; Jonasdottir, A.; Baker, A.; Thorleifsson, G.; Kristjansson, K.; et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 2007, 448, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Sinner, M.F.; Tucker, N.R.; Lunetta, K.L.; Ozaki, K.; Smith, J.G.; Trompet, S.; Bis, J.C.; Lin, H.; Chung, M.K.; Nielsen, J.B.; et al. Integrating genetic, transcriptional, and functional analyses to identify 5 novel genes for atrial fibrillation. Circulation 2014, 130, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Lenth, R.V. Java Applets for Power and Sample Size [computer software]. Available online: http://www.stat.uiowa.edu/~rlenth/Power (accessed on 3 February 2015).
- Mukherjee, R.; Herron, A.R.; Lowry, A.S.; Stroud, R.E.; Stroud, M.R.; Wharton, J.M.; Ikonomidis, J.S.; Crumbley, A.J., 3rd; Spinale, F.G.; Gold, M.R. Selective induction of matrix metalloproteinases and tissue inhibitor of metalloproteinases in atrial and ventricular myocardium in patients with atrial fibrillation. Am. J. Cardiol. 2006, 97, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cui, G.; Esmailian, F.; Plunkett, M.; Marelli, D.; Ardehali, A.; Odim, J.; Laks, H.; Sen, L. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation 2004, 109, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Anne, W.; Willems, R.; Roskams, T.; Sergeant, P.; Herijgers, P.; Holemans, P.; Ector, H.; Heidbuchel, H. Matrix metalloproteinases and atrial remodeling in patients with mitral valve disease and atrial fibrillation. Cardiovasc. Res. 2005, 67, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhong, M.; Yang, G.R.; Li, J.P.; Guo, C.; Wang, Z.; Zhang, Y. Matrix metalloproteinase-9/tissue inhibitors of metalloproteinase-1 expression and atrial structural remodeling in a dog model of atrial fibrillation: Inhibition with angiotensin-converting enzyme. Cardiovasc. Pathol. 2008, 17, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Yaghooti, H.; Firoozrai, M.; Fallah, S.; Khorramizadeh, M.R. Angiotensin ii differentially induces matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 production and disturbs MMP/TIMP balance. Avicenna J. Med. Biotechnol. 2010, 2, 79–85. [Google Scholar] [PubMed]
- Lauer, D.; Slavic, S.; Sommerfeld, M.; Thone-Reineke, C.; Sharkovska, Y.; Hallberg, A.; Dahlof, B.; Kintscher, U.; Unger, T.; Steckelings, U.M.; et al. Angiotensin type 2 receptor stimulation ameliorates left ventricular fibrosis and dysfunction via regulation of tissue inhibitor of matrix metalloproteinase 1/matrix metalloproteinase 9 axis and transforming growth factor β1 in the rat heart. Hypertension 2014, 63, e60–e67. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.V.; Sood, A.K.; Chen, M.; Li, Y.; Eubank, T.D.; Marsh, C.B.; Jewell, S.; Flavahan, N.A.; Morrison, C.; Yeh, P.E.; et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006, 66, 10357–10364. [Google Scholar] [CrossRef] [PubMed]
- Annabi, B.; Lachambre, M.P.; Plouffe, K.; Moumdjian, R.; Beliveau, R. Propranolol adrenergic blockade inhibits human brain endothelial cells tubulogenesis and matrix metalloproteinase-9 secretion. Pharmacol. Res. 2009, 60, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Murono, S.; Yoshizaki, T.; Sato, H.; Takeshita, H.; Furukawa, M.; Pagano, J.S. Aspirin inhibits tumor cell invasiveness induced by epstein-barr virus latent membrane protein 1 through suppression of matrix metalloproteinase-9 expression. Cancer Res. 2000, 60, 2555–2561. [Google Scholar] [PubMed]
- Tung, Y.C.; Wu, L.S.; Chen, W.J.; Kuo, C.T.; Wang, C.L.; Chang, C.J.; Tsai, H.Y.; Yeh, Y.H.; Hsu, L.A. C-Reactive Protein Gene Polymorphisms and the Risk of Atrial Fibrillation in a Chinese Population in Taiwan. Acta Cardiol. Sin. 2013, 29, 208–216. [Google Scholar]
- Hsu, L.A.; Yeh, Y.H.; Kuo, C.T.; Chen, Y.H.; Chang, G.J.; Tsai, F.C.; Chen, W.J. Microsatellite polymorphism in the heme oxygenase-1 gene promoter and the risk of atrial fibrillation in Taiwanese. PLoS ONE 2014, 9, e108773. [Google Scholar] [CrossRef] [PubMed]
- Claycomb, W.C.; Lanson, N.A., Jr.; Stallworth, B.S.; Egeland, D.B.; Delcarpio, J.B.; Bahinski, A.; Izzo, N.J., Jr. Hl-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. USA 1998, 95, 2979–2984. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Controls (n = 240) | AF Patients (n = 200) | p |
|---|---|---|---|
| Age, years | 55.7 ± 7.6 | 56.9 ± 8.4 | 0.14 |
| Gender (M/F) | 172/68 | 145/55 | 0.85 |
| BMI, kg/m2 | 25.3 ± 3.2 | 25.5 ± 4.5 | 0.68 |
| Hypertension, n (%) | 126 (52.5) | 119 (59.5) | 0.14 |
| Diabetes mellitus, n (%) | 17 (7.1) | 21 (10.5) | 0.20 |
| Smoking, n (%) | 62 (25.8) | 45 (22.6) | 0.43 |
| Hypercholesterolemia, n (%) | 25 (10.4) | 21 (10.5) | 0.98 |
| CAD, n (%) | 5 (2.1) | 10 (5.0) | 0.09 |
| Paroxysmal/Persistent, n (%) | – | 109/91 (54.5/45.5) | – |
| LA dimension > 40 mm, n (%) | – | 86 (43.0) | – |
| ARB, n (%) | 65 (27.1) | 83 (41.5) | <0.001 |
| ACE inhibitor, n (%) | 15 (6.2) | 11 (5.5) | 0.74 |
| β-Blocker, n (%) | 56 (23.3) | 88 (44.0) | <0.001 |
| Calcium antagonist, n (%) | 71 (29.6) | 79 (39.5) | 0.03 |
| Diuretic, n (%) | 11 (4.6) | 32 (16.0) | <0.001 |
| Digoxin, n (%) | 0 (0.0) | 34 (17.0) | <0.001 |
| Statin, n (%) | 41 (17.1) | 59 (29.5) | 0.002 |
| Aspirin, n (%) | 17 (7.1) | 85 (42.5) | <0.001 |
| Oral anticoagulant, n (%) | 0 (0.0) | 38 (19.0) | <0.001 |
| rs3918242 | Controls (n = 236) | AF Patients (n = 198) | p |
|---|---|---|---|
| Genotype | |||
| TT | 1 (0.4%) | 3 (1.5%) | 0.190 |
| CT | 55 (23.3%) | 35 (17.7%) | |
| CC | 180 (76.3%) | 160 (80.8%) | |
| Allele | |||
| T/C | 12.1/87.9 | 10.4/89.6 | 0.424 |
| No. | Age (Years) | Sex | rs3918242 Genotype | Underlying Cardiac Disease | DM | Hypertension | LV Ejection Fraction (%) | LAD (mm) | Previous Medical History | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAD | Operative Indication | Duration of AF (Years) | β Blocker | Digitalis | Statins | Diuretics | ACE Inhibitors or ARB | Calcium Channel Blockers | |||||||||
| SR | |||||||||||||||||
| 1 | 69 | M | C/C | – | AR | – | + | + | 71 | 43 | – | – | – | – | – | – | |
| 2 | 61 | M | C/C | – | AS + MS | – | – | + | 68 | 47 | – | – | + | + | + | – | |
| 3 | 79 | M | C/C | + | CABG | – | – | + | 65 | 31 | + | – | – | + | – | – | |
| AF | |||||||||||||||||
| 1 | 55 | F | C/T | – | AS + MS | 6 | – | – | 74 | 56 | – | + | – | + | + | – | |
| 2 | 64 | F | C/T | – | MS | 3 | – | – | 68 | 56 | – | – | – | + | + | – | |
| 3 | 59 | F | C/T | – | MS | 9 | – | – | 69 | 65 | – | – | – | – | – | – | |
| 4 | 49 | M | C/C | – | MS | <1 | – | + | 60 | 54 | – | – | – | – | – | – | |
| 5 | 48 | F | C/C | – | MR | 5 | – | + | 70 | 59 | + | – | – | + | – | – | |
| 6 | 62 | F | C/C | – | MS | 4 | – | – | 66 | 50 | + | + | – | – | – | – | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, F.-C.; Yeh, Y.-H.; Chen, W.-J.; Chan, Y.-H.; Kuo, C.-T.; Wang, C.-L.; Chang, C.-J.; Tsai, H.-Y.; Tsai, F.-C.; Hsu, L.-A. MMP9 Rs3918242 Polymorphism Affects Tachycardia-Induced MMP9 Expression in Cultured Atrial-Derived Myocytes but Is Not a Risk Factor for Atrial Fibrillation among the Taiwanese. Int. J. Mol. Sci. 2016, 17, 521. https://doi.org/10.3390/ijms17040521
Hsiao F-C, Yeh Y-H, Chen W-J, Chan Y-H, Kuo C-T, Wang C-L, Chang C-J, Tsai H-Y, Tsai F-C, Hsu L-A. MMP9 Rs3918242 Polymorphism Affects Tachycardia-Induced MMP9 Expression in Cultured Atrial-Derived Myocytes but Is Not a Risk Factor for Atrial Fibrillation among the Taiwanese. International Journal of Molecular Sciences. 2016; 17(4):521. https://doi.org/10.3390/ijms17040521
Chicago/Turabian StyleHsiao, Fu-Chih, Yung-Hsin Yeh, Wei-Jan Chen, Yi-Hsin Chan, Chi-Tai Kuo, Chun-Li Wang, Chi-Jen Chang, Hsin-Yi Tsai, Feng-Chun Tsai, and Lung-An Hsu. 2016. "MMP9 Rs3918242 Polymorphism Affects Tachycardia-Induced MMP9 Expression in Cultured Atrial-Derived Myocytes but Is Not a Risk Factor for Atrial Fibrillation among the Taiwanese" International Journal of Molecular Sciences 17, no. 4: 521. https://doi.org/10.3390/ijms17040521
APA StyleHsiao, F. -C., Yeh, Y. -H., Chen, W. -J., Chan, Y. -H., Kuo, C. -T., Wang, C. -L., Chang, C. -J., Tsai, H. -Y., Tsai, F. -C., & Hsu, L. -A. (2016). MMP9 Rs3918242 Polymorphism Affects Tachycardia-Induced MMP9 Expression in Cultured Atrial-Derived Myocytes but Is Not a Risk Factor for Atrial Fibrillation among the Taiwanese. International Journal of Molecular Sciences, 17(4), 521. https://doi.org/10.3390/ijms17040521