Cell and Signal Components of the Microenvironment of Bone Metastasis Are Affected by Hypoxia
Abstract
:1. Introduction
1.1. The Bone Marrow Is the “Metastatic Microenvironment”
1.2. Interaction of Megakaryocytes with Functional Niches in Bone Metastasis
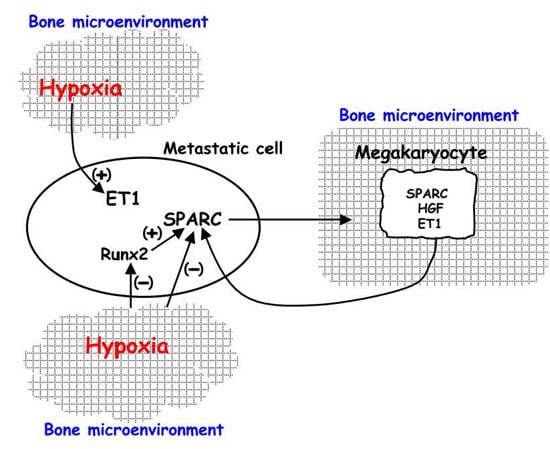
2. Hypoxia Affects the Response to Environmental Biological Stimuli Regulating Endothelin 1 (ET-1)/ETAR and Hepatocyte Growth Factor (HGF)/Met Receptor Axes in Bone Metastasis
3. Regulation of HIF-1 Activity in Metastatic Cells and in the Xenograft Model: The Influence on Pre-Metastatic Niche Formation
4. HIF-1 Activity in Human Bone Metastasis and Therapy
Acknowledgments
Conflicts of Interest
Abbreviations
| SPARC | Secreted protein acidic and rich in cysteine |
| HSCs | Hematopoietic stem cells |
| CTCs | Circulating tumor cells |
| EMT | Epithelial-mesenchymal transition |
| ET-1 | Endothelin-1 |
| HGF | Hepatocyte growth factor |
| HIF-1 | Hypoxia inducible factor-1 |
| Wwox | WWdomain-containing oxidoreductase |
| MET | Mesenchymal-epithelial transition |
| LOX | Lysyl oxidase |
| CSC | Cancer stem cells |
| TAZ | Transcriptional co-activator with PDZ-binding motif |
| TNBC | Triple negative breast cancer |
| TEPs | Tumor-educated blood platelets |
References
- Kaplan, R.N.; Psaila, B.; Lyden, D. Bone marrow cells in the “pre-metastatic niche”: Within bone and beyond. Cancer Metastasis Rev. 2006, 25, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Psaila, B.; Lyden, D.; Roberts, I. Megakaryocytes, malignancy and bone marrow vascular niches. J. Thromb. Haemost. 2012, 10, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim. Biophys. Acta 2016, 1863, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xing, Q.; Qian, Z.; Tahtinen, M.; Zhang, Z.; Shearier, E.; Qi, S.; Zhao, F. Hypoxia created human mesenchymal stem cell sheet for prevascularized 3D tissue construction. Adv. Health Mater. 2016, 5, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Parks, S.K.; Cormerais, Y.; Marchiq, I.; Pouyssegur, J. Hypoxia optimises tumor growth by controlling nutrient import and acidic metabolite export. Mol. Asp. Med. 2016, 47–48, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Kang, Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacol. Ther. 2014, 141, 222–233. [Google Scholar] [CrossRef] [PubMed]
- El-Haibi, C.P.; Karnoub, A.E. Mesenchymal stem cells in the pathogenesis and therapy of breast cancer. J. Mammary Gland Biol. Neoplasia 2010, 15, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.M.; Vallone, V.B.; Labovsky, V.; Choi, H.; Hofer, E.L.; Feldman, L.; Bordenave, R.H.; Batagelj, E.; Dimase, F.; Villafañe, A.R.; et al. Changes in the peripheral blood and bone marrow from untreated advanced breast cancer patients that are associated with the establishment of bone metastases. Clin. Exp. Metastasis 2014, 31, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Dieudonné, S.C.; Xu, T.; Chou, J.Y.; Kutznetsov, S.A.; Satomura, K.; Mankani, M.; Fedarko, N.S.; Smith, E.P.; Robey, P.G.; Young, M.F. Immortalization and characterization of bone marrow stromal fibroblasts from a patient with a loss of function mutation in the estrogen receptor-α gene. J. Bone Miner. Res. 1998, 13, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Brekken, R.A.; Sage, E.H. SPARC, a matricellular protein: At the crossroads of cell-matrix communication. Matrix Biol. 2001, 19, 816–827. [Google Scholar] [CrossRef]
- Kacena, M.A.; Gundberg, C.M.; Horowitz, M.C. A reciprocal regulatory interaction between megakaryocytes, bone cells, and hematopoietic stem cells. Bone 2006, 39, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Weinberg, R.A. Epithelial-mesenchymal plasticity: A central regulator of cancer progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Desiderio, M.A. Hepatocyte growth factor in invasive growth of carcinomas. Cell. Mol. Life Sci. 2007, 64, 1341–1354. [Google Scholar] [CrossRef] [PubMed]
- Bendinelli, P.; Maroni, P.; Matteucci, E.; Desiderio, M.A. HGF and TGFβ1 differently influenced Wwox regulatory function on Twist program for mesenchymal-epithelial transition in bone metastatic versus parental breast carcinoma cells. Mol. Cancer 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Gheldof, A.; Tatari, M.; Christofori, G. EMT as the ultimate survival mechanism of cancer cells. Semin. Cancer Biol. 2012, 22, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.B.; Giaccia, A.J. Hypoxic control of metastasis. Science 2016, 352, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Maroni, P.; Bendinelli, P.; Matteucci, E.; Locatelli, A.; Nakamura, T.; Scita, G.; Desiderio, M.A. Osteolytic bone metastasis is hampered by impinging on the interplay among autophagy, anoikis and ossification. Cell Death Dis. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Bendinelli, P.; Maroni, P.; Matteucci, E.; Luzzati, A.; Perrucchini, G.; Desiderio, M.A. Microenvironmental stimuli affect Endothelin-1 signaling responsible for invasiveness and osteomimicry of bone metastasis from breast cancer. Biochim. Biophys. Acta 2014, 1843, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Rosanò, L.; Spinella, F.; Bagnato, A. Endothelin 1 in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2013, 13, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, E.; Maroni, P.; Disanza, A.; Bendinelli, P.; Desiderio, M.A. Coordinate regulation of microenvironmental stimuli and role of methylation in bone metastasis from breast carcinoma. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Maroni, P.; Bendinelli, P.; Morelli, D.; Drago, L.; Luzzati, A.; Perrucchini, G.; Bonini, C.; Matteucci, E.; Desiderio, M.A. High SPARC expression starting from dysplasia, associated with breast carcinoma, is predictive for bone metastasis without enhancement of plasma levels. Int. J. Mol. Sci. 2015, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, C.; Gao, X.; Welte, T.; Muscarella, A.M.; Tian, L.; Zhao, H.; Zhao, Z.; Du, S.; Tao, J.; et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 2015, 27, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumor cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.-L.; Sun, J.; Gong, S.-Q.; Yu, X.-F.; Gong, R.; Deng, H. Interaction between circulating cancer cells and platelets: Clinical implication. Clin. J. Cancer Res. 2015, 27, 450–460. [Google Scholar]
- Andrade, S.S.; Gouvea, I.E.; Silva, M.C.C.; Dognani Castro, E.; de Paula, C.A.A.; Okamoto, D.; Oliveira, L.; Peres, G.B.; Ottaiano, T.; Facina, G.; et al. Cathepsin K induces platelet dysfunction and affects cell signaling in breast cancer—Molecularly distinct behavior of cathepsin K in breast cancer. BMC Cancer 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.-H.; Bhoo-Pathy, N.; Ng, K.-L.; Jabir, R.S.; Tan, G.H.; See, M.-H.; Jamaris, S.; Taib, N.A. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br. J. Cancer 2015, 113, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Joosse, S.A.; Pantel, K. Tumor-educated platelets as liquid biopsy in cancer patients. Cancer Cell 2015, 28, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Bendinelli, P.; Maroni, P.; Matteucci, E.; Luzzati, A.; Perrucchini, G.; Desiderio, M.A. Hypoxia inducible factor-1 is activated by transcriptional co-activator with PDZ-binding motif (TAZ) versus WWdomain-containing oxidoreductase (WWOX) in hypoxic microenvironment of bone metastasis from breast cancer. Eur. J. Cancer 2013, 49, 2608–2618. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Z.; Hou, Y.; Fang, W. Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am. J. Transl. Res. 2015, 7, 2527–2535. [Google Scholar] [PubMed]
- Barnes, G.L.; Javed, A.; Waller, S.M.; Kamal, M.H.; Hebert, K.E.; Hassan, M.Q.; Bellahcene, A.; van Wijnen, A.J.; Young, M.F.; Lian, J.B.; et al. Osteoblast-related transcription factors Runx2 (Cbfa1/AML3) and MSX2 mediate the expression of bone sialoprotein in human metastatic breast cancer cells. Cancer Res. 2003, 63, 2631–2637. [Google Scholar] [PubMed]
- Pratap, J.; Javed, A.; Languino, L.R.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol. Cell. Biol. 2005, 25, 8581–8591. [Google Scholar] [CrossRef] [PubMed]
- Previdi, S.; Maroni, P.; Matteucci, E.; Broggini, M.; Bendinelli, P.; Desiderio, M.A. Interaction between human-breast cancer metastasis and bone microenvironment through activated hepatocyte growth factor/Met and β-catenin/Wnt pathways. Eur. J. Cancer 2010, 46, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Pennacchietti, S.; Michieli, P.; Galluzzo, M.; Mazzone, M.; Giordano, S.; Comoglio, P.M. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 2003, 3, 347–361. [Google Scholar] [CrossRef]
- Taddei, M.L.; Giannoni, E.; Fiaschi, T.; Chiarugi, P. Anoikis: An emerging hallmark in health and diseases. J. Pathol. 2012, 226, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Mukhopadhyay, S.; Das, D.N.; Sinha, N.; Naik, P.P.; Bhutia, S.K. Mechanism of autophagic regulation in carcinogenesis and cancer therapeutics. Semin. Cell Dev. Biol. 2015, 39, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Maroni, P.; Matteucci, E.; Luzzati, A.; Perrucchini, G.; Bendinelli, P.; Desiderio, M.A. Nuclear co-localization and functional interaction of COX-2 and HIF-1α characterize bone metastasis of human breast carcinoma. Breast Cancer Res. Treat. 2011, 129, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Maroni, P.; Matteucci, E.; Drago, L.; Banfi, G.; Bendinelli, P.; Desiderio, M.A. Hypoxia induced E-cadherin involving regulators of Hippo pathway due to HIF-1α stabilization/nuclear translocation in bone metastasis from breast carcinoma. Exp. Cell Res. 2015, 330, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Tomida, A.; Tsuruo, T. Dephosphorylated hypoxia-inducible factor 1α as a mediator of p53-dependent apoptosis during hypoxia. Oncogene 2001, 20, 5779–5788. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, T.; Kizaka-Kondoh, S.; Hirota, K.; Hiraoka, M.; Yoneda, T. Hypoxia and hypoxia-inducible factor 1 expression enhance osteolytic bone metastases of breast cancer. Cancer Res. 2007, 67, 4157–4163. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Rumney, R.M.; Schoof, E.M.; Perryman, L.; Høye, A.M.; Agrawal, A.; Bird, D.; Latif, N.A.; Forrest, H.; Evans, H.R.; et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 2015, 522, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Erler, J.T.; Bennewith, K.L.; Cox, T.R.; Lang, G.; Bird, D.; Koong, A.; Le, Q.T.; Giaccia, A.J. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 2009, 15, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Dales, J.P.; Garcia, S.; Meunier-Carpentier, S.; Andrac-Meyer, L.; Haddad, O.; Lavaut, M.N.; Allasia, C.; Bonnier, P.; Charpin, C. Overexpression of hypoxia-inducible factor HIF-1α predicts early relapse in breast cancer: Retrospective study in a series of 745 patients. Int. J. Cancer 2005, 116, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Samanta, D.; Xiang, L.; Zhang, H.; Hu, H.; Chen, I.; Bullen, J.W.; Semenza, G.L. Chemotherapy triggers HIF-1-dependent glutathione synthesis and copper chelation that induces the breast cancer stem cell phenotype. Proc. Natl. Acad. Sci. USA 2015, 112, E4600–E4609. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W.; Schipani, E.; Giaccia, A.J. HIF-1 targets in bone remodeling and metastatic disease. Pharm. Ther. 2015, 150, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Clément-Demange, L.; Clézardin, P. Emerging therapies in bone metastasis. Curr. Opin. Pharmacol. 2015, 22, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, F.; Silvestris, F.; Ibrahim, T.; Cognetti, F.; Generali, D.; Ripamonti, C.I.; Amadori, D.; Colleoni, M.A.; Conte, P.; del Mastro, L.; et al. Targeting bone metastatic cancer: Role of the mTOR pathway. Biochim. Biophys. Acta 2014, 1845, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, L.U.; Thulin, M.H.; Plas, P.; Olsson, A.; Damber, J.-E.; Welén, K. Tasquinimod inhibits prostate cancer growth in bone through alterations in the bone microenvironment. Prostate 2016, 76, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Lee, K.K.; Zhang, L.; Gerton, J.L. Stimulation of mTORC1 with l-leucine rescues defects associated with Roberts syndrome. PLoS Genet. 2013, 9, e1003857. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Gogol, M.; Gaudenz, K.; Gerton, J.L. Improved transcription and translation with l-leucine stimulation of mTORC1 in Roberts syndrome. BMC Genom. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Guba, M.; von Breitenbuch, P.; Steinbauer, M.; Koehl, G.; Flegel, S.; Hornung, M.; Bruns, C.J.; Zuelke, C.; Farkas, S.; Anthuber, M.; et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: Involvement of vascular endothelial growth factor. Nat. Med. 2002, 8, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A., III; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Gnant, M.; Baselga, J.; Rugo, H.S.; Noguchi, S.; Burris, H.A.; Piccart, M.; Hotobagyi, G.N.; Eakle, J.; Mukai, H.; Iwata, H.; et al. Effect of everolimus on bone marker levels and progressive disease in bone in BOLERO-2. J. Natl. Cancer Inst. 2013, 105, 654–663. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendinelli, P.; Maroni, P.; Matteucci, E.; Desiderio, M.A. Cell and Signal Components of the Microenvironment of Bone Metastasis Are Affected by Hypoxia. Int. J. Mol. Sci. 2016, 17, 706. https://doi.org/10.3390/ijms17050706
Bendinelli P, Maroni P, Matteucci E, Desiderio MA. Cell and Signal Components of the Microenvironment of Bone Metastasis Are Affected by Hypoxia. International Journal of Molecular Sciences. 2016; 17(5):706. https://doi.org/10.3390/ijms17050706
Chicago/Turabian StyleBendinelli, Paola, Paola Maroni, Emanuela Matteucci, and Maria Alfonsina Desiderio. 2016. "Cell and Signal Components of the Microenvironment of Bone Metastasis Are Affected by Hypoxia" International Journal of Molecular Sciences 17, no. 5: 706. https://doi.org/10.3390/ijms17050706
APA StyleBendinelli, P., Maroni, P., Matteucci, E., & Desiderio, M. A. (2016). Cell and Signal Components of the Microenvironment of Bone Metastasis Are Affected by Hypoxia. International Journal of Molecular Sciences, 17(5), 706. https://doi.org/10.3390/ijms17050706