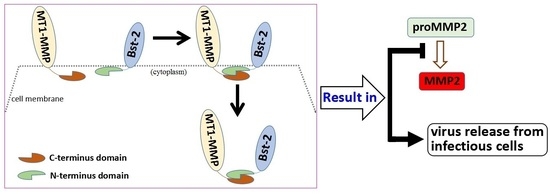
MT1-MMP Inhibits the Activity of Bst-2 via Their Cytoplasmic Domains Dependent Interaction
Abstract
:1. Introduction
2. Results
2.1. Induced Expression of Bst-2 in HT1080 Cells and Its Activity Inhibition by MT1-MMP in Both MDCK and HT1080 Cells
2.2. Expression of Bst-2 Including mRNA Level and Protein Level Was Not Affected by MT1-MMP
2.3. Interaction and Co-Localization Happened between Proteins Bst-2 and MT1-MMP
2.4. N-Terminal Domain (NT Domain) of Bst-2 Was Critical in Regulating the Activity of Bst-2 Itself and in Bst-2 Interacting with MT1-MMP
2.5. Importance of the C-Terminal Domain of MT1-MMP in Interacting with Bst-2 and Inhibiting the Tetherin Activity of Bst-2
2.6. Role of the N-Terminal Domain of Bst-2 in Down-Regulating the Activity of MT1-MMP
2.7. Discussion
3. Materials and Methods
3.1. Cell Culture and Transfection
3.2. Plasmids, Antibodies and Chemicals
3.3. Zymography, Western Blotting and Immunoprecipitation
3.4. Reverse Transcription PCR (RT-PCR) and Quantitative Real-Time PCR (qPCR)
3.5. Growth of MDCK and HT1080 Cells in Three-Dimensional Type I Collagen Lattice
3.6. Migration Assay of MDCK and HT1080 Cells
3.7. Immunostaining and Confocal Microscopy Assay
3.8. Virion-Release Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuhl, B.D.; Cheng, V.; Wainberg, M.A.; Liang, C. Tetherin and its viral antagonists. J. Neuroimmune Pharmacol. 2011, 6, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Kupzig, S.; Korolchuk, V.; Rollason, R.; Sugden, A.; Wilde, A.; Banting, G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 2003, 4, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Neil, S.J.; Zang, T.; Bieniasz, P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 2008, 451, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, N.; Goff, D.; Katsura, C.; Jorgenson, R.L.; Mitchell, R.; Johnson, M.C.; Stephens, E.B.; Guatelli, J. The interferon-induced protein Bst-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 2008, 3, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.T.; Serra-Moreno, R.; Singh, R.K.; Guatelli, J.C. BST-2/tetherin: A new component of the innate immune response to enveloped viruses. Trends Microbiol. 2010, 18, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.F.; Heyer, L.N.; von Bredow, B.; Weisgrau, K.L.; Moldt, B.; Burton, D.R.; Rakasz, E.G.; Evans, D.T. Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity. Proc. Natl. Acad. Sci. USA 2014, 111, 6425–6430. [Google Scholar] [CrossRef] [PubMed]
- Rollason, R.; Korolchuk, V.; Hamilton, C.; Schu, P.; Banting, G. Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J. Cell Sci. 2007, 120, 3850–3858. [Google Scholar] [CrossRef] [PubMed]
- Rollason, R.; Korolchuk, V.; Hamilton, C.; Jepson, M.; Banting, G. A CD317/tetherin-RICH2 complex plays a critical role in the organization of the subapical actin cytoskeleton in polarized epithelial cells. J. Cell Biol. 2009, 184, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Seiki, M. The cell surface: The stage for matrix metalloproteinase regulation of migration. Curr. Opin. Cell Biol. 2002, 14, 624–632. [Google Scholar] [CrossRef]
- Seiki, M. Membrane-type 1 matrix metalloproteinase: A key enzyme for tumor invasion. Cancer Lett. 2003, 194, 1–11. [Google Scholar] [CrossRef]
- Hiraoka, N.; Allen, E.; Apel, I.J.; Gyetko, M.R.; Weiss, S.J. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell 1998, 95, 365–377. [Google Scholar] [CrossRef]
- Zhou, Z.; Apte, S.S.; Soininen, R.; Cao, R.; Baaklini, G.Y.; Rauser, R.W.; Wang, J.; Cao, Y.; Tryggvason, K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. USA 2000, 97, 4052–4057. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, E.; Imai, K.; Fujii, Y.; Sato, H.; Seiki, M.; Okada, Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J. Biol. Chem. 1997, 272, 2446–2451. [Google Scholar] [PubMed]
- D’ortho, M.P.; Will, H.; Atkinson, S.; Butler, G.; Messent, A.; Gavrilovic, J.; Smith, B.; Timpl, R.; Zardi, L.; Murphy, G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur. J. Biochem. 1997, 250, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Hotary, K.; Allen, E.; Punturieri, A.; Yana, I.; Weiss, S.J. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J. Cell Biol. 2000, 149, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Lehti, K.; Lohi, J.; Juntunen, M.M.; Pei, D.; Keski-Oja, J. Oligomerization through hemopexin and cytoplasmic domains regulates the activity and turnover of membrane-type 1 matrix metalloproteinase. J. Biol. Chem. 2002, 277, 8440–8448. [Google Scholar] [CrossRef] [PubMed]
- Koshikawa, N.; Giannelli, G.; Cirulli, V.; Miyazaki, K.; Quaranta, V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J. Cell Biol. 2000, 148, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Will, H.; Atkinson, S.J.; Butler, G.S.; Smith, B.; Murphy, G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J. Biol. Chem. 1996, 271, 17119–17123. [Google Scholar] [CrossRef] [PubMed]
- Bigg, H.F.; Morrison, C.J.; Butler, G.S.; Bogoyevitch, M.A.; Wang, Z.; Soloway, P.D.; Overall, C.M. Tissue inhibitor of metalloproteinases-4 inhibits but does not support the activation of gelatinase A via efficient inhibition of membrane type 1-matrix metalloproteinase. Cancer Res. 2001, 61, 3610–3618. [Google Scholar] [PubMed]
- Gálvez, B.G.; Matías-Román, S.; Yáñez-Mó, M.; Vicente-Manzanares, M.; Sánchez-Madrid, F.; Arroyo, A.G. Caveolae are a novel pathway for membrane-type 1 matrix metalloproteinase traffic in human endothelial cells. Mol. Biol. Cell 2004, 15, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Lehti, K.; Wang, X.; Weiss, S.J.; Keski-Oja, J.; Pei, D. Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc. Natl. Acad. Sci. USA 2001, 98, 13693–13698. [Google Scholar] [CrossRef] [PubMed]
- Uekita, T.; Itoh, Y.; Yana, I.; Ohno, H.; Seiki, M. Cytoplasmic tail-dependent internalization of membrane-type 1 matrix metalloproteinase is important for its invasion-promoting activity. J. Cell Biol. 2001, 155, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Remacle, A.; Murphy, G.; Roghi, C. Membrane type I-matrix metalloproteinase (MT1-MMP) is internalised by two different pathways and is recycled to the cell surface. J. Cell Sci. 2003, 116, 3905–3916. [Google Scholar] [CrossRef] [PubMed]
- Nyalendo, C.; Michaud, M.; Beaulieu, E.; Roghi, C.; Murphy, G.; Gingras, D.; Béliveau, R. Src-dependent phosphorylation of membrane type I matrix metalloproteinase on cytoplasmic tyrosine 573: Role in endothelial and tumor cell migration. J. Biol. Chem. 2007, 282, 15690–15699. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Cordero, J.J.; Marrero-Diaz, R.; Megías, D.; Genís, L.; García-Grande, A.; García, M.A.; Arroyo, A.G.; Montoya, M.C. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 2007, 26, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Hlubek, F.; Spaderna, S.; Jung, A.; Kirchner, T.; Brabletz, T. β-Catenin activates a coordinated expression of the proinvasive factors laminin-5 γ2 chain and MT1-MMP in colorectal carcinomas. Int. J. Cancer 2004, 108, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki-Raby, B.; Gilles, C.; Polette, M.; Martinella-Catusse, C.; Bonnet, N.; Puchelle, E.; Foidart, J.M.; Van Roy, F.; Birembaut, P. E-Cadherin mediates MMP down-regulation in highly invasive bronchial tumor cells. Am. J. Pathol. 2003, 163, 653–661. [Google Scholar] [CrossRef]
- Liu, P.; Yang, J.; Pei, J.; Pei, D.; Wilson, M.J. Regulation of MT1-MMP activity by β-catenin in MDCK non-cancer and HT1080 cancer cells. J. Cell Physiol. 2010, 225, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Zhao, D.; Yin, Z.; Liu, P. BST-2 binding with cellular MT1-MMP blocks cell growth and migration via decreasing MMP2 activity. J. Cell Biochem. 2012, 113, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Jia, X.; McNatt, M.W.; Zang, T.; Pan, B.; Meng, W.; Wang, H.-W.; Bieniasz, P.D.; Xiong, Y. Structural insight into the mechanisms of enveloped virus tethering by tetherin. Proc. Natl. Acad. Sci. USA 2010, 107, 18428–18432. [Google Scholar]
- Poincloux, R.; Lizárraga, F.; Chavrier, P. Matrix invasion by tumour cells: A focus on MT1-MMP trafficking to invadopodia. J. Cell Sci. 2009, 122, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Schubert, H.L.; Zhai, Q.; Sandrin, V.; Eckert, D.M.; Garcia-Maya, M.; Saul, L.; Sundquist, W.I.; Steiner, R.A.; Hill, C.P. Structural and functional studies on the extracellular domain of Bst2/tetherin in reduced and oxidized conformations. Proc. Natl. Acad. Sci. USA 2010, 107, 17951–17956. [Google Scholar] [CrossRef] [PubMed]
- Pei, D. Identification and characterization of the fifth membrane-type matrix metalloproteinase MT5-MMP. J. Biol. Chem. 1999, 274, 8925–8932. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Weiss, S.J. Transmembrane-deletion mutants of the membrane-type matrix metalloproteinase-1 process progelatinase A and express intrinsic matrix-degrading activity. J. Biol. Chem. 1996, 271, 9135–9140. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Pei, D. Distinct roles of catalytic and pexin-like domains in membrane-type matrix metalloproteinase (MMP)-mediated pro-MMP-2 activation and collagenolysis. J. Biol. Chem. 2003, 278, 38765–38771. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Yi, J.; Yang, W.; Wang, X.; Jiang, A.; Pei, D. Functional characterization of MT3-MMP in transfected MDCK cells: Progelatinase A activation and tubulogenesis in 3-D collagen lattice. FASEB J. 2000, 14, 2559–2568. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Liu, L.; Zhu, C.; Zhu, Q.; Lu, S.; Liu, P. MT1-MMP Inhibits the Activity of Bst-2 via Their Cytoplasmic Domains Dependent Interaction. Int. J. Mol. Sci. 2016, 17, 818. https://doi.org/10.3390/ijms17060818
Fan L, Liu L, Zhu C, Zhu Q, Lu S, Liu P. MT1-MMP Inhibits the Activity of Bst-2 via Their Cytoplasmic Domains Dependent Interaction. International Journal of Molecular Sciences. 2016; 17(6):818. https://doi.org/10.3390/ijms17060818
Chicago/Turabian StyleFan, Long, Li Liu, Cuicui Zhu, Qingyi Zhu, Shan Lu, and Ping Liu. 2016. "MT1-MMP Inhibits the Activity of Bst-2 via Their Cytoplasmic Domains Dependent Interaction" International Journal of Molecular Sciences 17, no. 6: 818. https://doi.org/10.3390/ijms17060818
APA StyleFan, L., Liu, L., Zhu, C., Zhu, Q., Lu, S., & Liu, P. (2016). MT1-MMP Inhibits the Activity of Bst-2 via Their Cytoplasmic Domains Dependent Interaction. International Journal of Molecular Sciences, 17(6), 818. https://doi.org/10.3390/ijms17060818