TSPO Ligand-Methotrexate Prodrug Conjugates: Design, Synthesis, and Biological Evaluation
Abstract
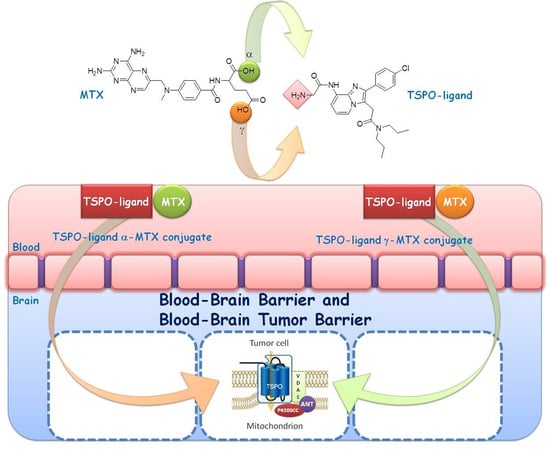
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Lipophilicity
2.3. Stability Studies
2.4. Radioligand Binding Assays
2.5. Cytotoxicity Studies
2.6. Transport Studies
3. Experimental Section
3.1. Materials and Methods
3.2. High-Performance Liquid Chromatography (HPLC) Analyses
3.3. Stability Studies in Phosphate Buffer Solution
3.4. Stability Studies in Rat Serum Solution
3.5. Synthesis of TSPO-Ligand-MTX Conjugates Prodrugs
3.6. Biology
3.6.1. Radioligand Binding Assays
3.6.2. Cell Cultures and Cell Viability Analysis by MTT Assay
3.6.3. Bi-Directional Permeability Study
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BBB | blood-brain barrier |
| BBTB | blood-brain tumor barrier |
| CBR | Central-type Benzodiazepine Receptor |
| CDI | 1,1′-carbonyldiimidazole |
| CNS | Central Nervous System |
| COSY | COrrelation SpectroscopY |
| EDAC | N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride |
| Gly | Glycine |
| Glu | Glutamic acid |
| MTX | Methotrexate |
| NOESY | Nuclear Overhauser Effect SpectroscopY |
| PBR | Peripheral-type Benzodiazepine Receptor |
| TEER | Transendothelial electrical resistance |
| TSPO | Translocator protein (18 kDa) |
References
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.J.; Limdermann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010, 9, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.; Moon, B.S.; Park, H.S.; Laquintana, V.; Jung, J.H.; Cutrignelli, A.; Lopedota, A.; Franco, M.; Kim, S.E.; Lee, B.C.; et al. A novel PET imaging probe for the detection and monitoring of translocator protein 18 kDa expression in pathological disorders. Sci. Rep. 2016, 6, 20422. [Google Scholar] [CrossRef] [PubMed]
- Midzak, A.; Zirkin, B.; Papadopoulos, V. Translocator protein: Pharmacology and steroidogenesis. Biochem. Soc. Trans. 2015, 43, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Midzak, A.; Denora, N.; Laquintana, V.; Cutrignelli, A.; Lopedota, A.; Franco, M.; Altomare, C.D.; Papadopoulos, V. 2-Phenylimidazo[1,2-a]pyridine-containing ligands of the 18-kDa translocator protein (TSPO) behave as agonists and antagonists of steroidogenesis in a mouse leydig tumor cell line. Eur. J. Pharm. Sci. 2015, 76, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.J.; Kahlert, J.; Kassiou, M.; Rendina, L.M. The translocator protein (TSPO): A novel target for cancer chemotherapy. Int. J. Biochem. Cell Biol. 2013, 45, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Werry, E.L.; Barron, M.L.; Kassiou, M. TSPO as a target for glioblastoma therapeutics. Biochem. Soc. Trans. 2015, 43, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Levin, E.; Weisinger, G.; Leschiner, S.; Spanier, I.; Snyder, S.H.; Weizman, A.; Gavish, M. Peripheral-type benzodiazepine receptor density and in vitro tumorigenicity of glioma cell lines. Biochem. Pharmacol. 2004, 68, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Rechichi, M.; Salvetti, A.; Chelli, B.; Costa, B.; da Pozzo, E.; Spinetti, F.; Lena, A.; Evangelista, M.; Rainaldi, G.; Martini, C.; et al. TSPO over-expression increases motility, transmigration and proliferation properties of C6 rat glioma cells. Biochim. Biophys. Acta 2008, 2, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Laquintana, V.; Pisu, M.G.; Dore, R.; Murru, L.; Latrofa, A.; Trapani, G.; Sanna, E. 2-Phenyl-imidazo[1,2-a]pyridine compounds containing hydrophilic groups as potent and selective ligands for peripheral benzodiazepine receptors: Synthesis, binding affinity and electrophysiological studies. J. Med. Chem. 2008, 51, 6876–6888. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Tripathi, R.; Mishra, B. Methotrexate: A detailed review on drug delivery and clinical aspects. Expert Opin. Drug Deliv. 2012, 9, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Kasim, N.A.; Whitehouse, M.; Ramachandran, C.; Bermejo, M.; Lennernäs, H.; Hussain, A.S.; Junginger, H.E.; Stavchansky, S.A.; Midha, K.K.; Shah, V.P.; et al. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol. Pharm. 2004, 1, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.A.; Sreevatsa, A.; Chinnagiriyappa, L.K.; Dasappa, L.; Suresh, T.M.; Babu, G. Methotrexate-induced chemical meningitis in patients with acute lymphoblastic leukemia/lymphoma. Ann. Indian Acad Neurol. 2015, 8, 206–209. [Google Scholar]
- Trapani, G.; Denora, N.; Trapani, A.; Laquintana, V. Recent advances in ligand targeted therapy. J. Drug Target. 2012, 1, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Laquintana, V.; Trapani, A.; Lopedota, A.; Latrofa, A.; Gallo, J.M.; Trapani, G. Translocator protein (TSPO) ligand-Ara-C (cytarabine) conjugates as a strategy to deliver antineoplastic drugs and to enhance drug clinical potential. Mol. Pharm. 2010, 7, 2255–2269. [Google Scholar] [CrossRef] [PubMed]
- Laquintana, V.; Denora, N.; Lopalco, A.; Lopedota, A.; Cutrignelli, A.; Lasorsa, F.M.; Agostino, G.; Franco, M. Translocator protein ligand-PLGA conjugated nanoparticles for 5-fluorouracil delivery to glioma cancer cells. Mol. Pharm. 2014, 11, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Laquintana, V.; Lopalco, A.; Iacobazzi, R.M.; Lopedota, A.; Cutrignelli, A.; Iacobellis, G.; Annese, C.; Cascione, M.; Leporatti, S.; et al. In vitro targeting and imaging the translocator protein TSPO 18-kDa through G(4)-PAMAM-FITC labeled dendrimer. J. Control. Release 2013, 172, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Laquintana, V.; Denora, N.; Musacchio, T.; Lasorsa, M.; Latrofa, A.; Trapani, G. Peripheral benzodiazepine receptor ligand-PLGA polymer conjugates potentially useful as delivery systems of apoptotic agents. J. Control. Release 2009, 137, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Annese, C.; Fanizza, I.; Calvano, C.D.; D’Accolti, L.; Fusco, C.; Curci, R.; Williard, P.G. Selective synthesis of hydroxy analogues of valinomycin using methyl(trifluoromethyl)dioxirane. Org. Lett. 2011, 13, 5096–5099. [Google Scholar] [CrossRef] [PubMed]
- Norinder, U.; Haeberlein, M. Computational approaches to the prediction of the blood-brain distribution. Adv. Drug Deliv. Rev. 2002, 54, 291–313. [Google Scholar] [CrossRef]
- Kreisl, W.C.; Jenko, J.K.; Hines, C.S.; Lyoo, H.C.; Corona, W.; Morse, C.L.; Zoghbi, S.S.; Hyde, T.; Kleinman, J.E.; Pike, V.W.; et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J. Cereb. Blood Flow Metab. 2013, 33, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Garberg, P.; Ball, M.; Borg, N.; Cecchelli, R.; Fenart, L.; Hurst, R.D.; Lindmark, T.; Mabondzo, A.; Nilsson, J.E.; Raub, T.J.; et al. In vitro models for the blood-brain barrier. Toxicol. Vitr. 2005, 19, 299–334. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yao, Y.; Tsirka, S.E.; Cao, Y. Cell-culture models of the blood-brain barrier. Stroke 2014, 45, 2514–2526. [Google Scholar] [CrossRef] [PubMed]
- Audus, K.L.; Borchardt, R.T. Bovine brain microvessel endothelial cell monolayers as a model system for the blood–brain barrier. Ann. N. Y. Acad. Sci. 1987, 507, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Cassano, T.; Laquintana, V.; Lopalco, A.; Trapani, A.; Cimmino, C.S.; Laconca, L.; Giuffrida, A.; Trapani, G. Novel codrugs with GABAergic activity for dopamine delivery in the brain. Int. J. Pharm. 2012, 437, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Laquintana, V.; Trapani, A.; Denora, N.; Wang, F.; Gallo, J.M.; Trapani, G. New strategies to deliver anticancer drugs to brain tumors. Expert Opin. Drug Deliv. 2009, 6, 1017–1032. [Google Scholar] [CrossRef] [PubMed]



| Compound | CLogP 1 | t1/2 (h) PBS | t1/2 (h) Diluted Rat Serum |
|---|---|---|---|
| 2 | +3.25 ± 1.10 | - | - |
| 3 | +5.21 ± 1.37 | 144 ± 25 | 4.1 ± 0.9 |
| 4 | +4.40 ± 1.37 | 74 ± 12 | 3.3 ± 0.5 |
| MTX | −0.24 ± 0.72 | - | - |
| Compound | IC50 (nM) | SI 3 | IC50 (nM) Rat Glioma Cells | IC50 (nM) Human Glioma Cells | Transport across MDCKII-MDR1 4 | |||
|---|---|---|---|---|---|---|---|---|
| TSPO Cortex | CBR Cerebral | C6 | RG2 | SF126 | SF188 | Papp (AP) (cm/s) | ||
| 2 1 | 24.0 ± 4.2 | >105 | >4.1 × 103 | - | - | - | - | - |
| 3 | 7.2 ± 2.1 | >105 | >1.4 × 104 | 1.2 ± 0.7 * | 4.2 ± 3.2 * | 14.2 ± 4.2 * | 9.2 ± 3.4 * | 3.65 ± 0.14 × 10−6 |
| 4 | 40.3 ± 3.8 | >105 | >2.5 × 103 | 2.2 ± 0.6 * | 15.2 ± 2.9 ** | 25.0 ± 2.6 ** | 18.2 ± 2.0 ** | 3.32 ± 0.28 × 10−6 |
| MTX | - | - | - | 2.6 ± 0.8 | 3.8 ± 1.5 | 1.2 ± 0.3 | 1.2 ± 0.2 | 9.10 ± 0.44 × 10−8 |
| PK11195 2 | 1.5 ± 1.5 | >105 | >6.7 × 104 | - | - | - | - | - |
| Flunitrazepam 2 | - | 5.1 ± 0.5 | - | - | - | - | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laquintana, V.; Denora, N.; Cutrignelli, A.; Perrone, M.; Iacobazzi, R.M.; Annese, C.; Lopalco, A.; Lopedota, A.A.; Franco, M. TSPO Ligand-Methotrexate Prodrug Conjugates: Design, Synthesis, and Biological Evaluation. Int. J. Mol. Sci. 2016, 17, 967. https://doi.org/10.3390/ijms17060967
Laquintana V, Denora N, Cutrignelli A, Perrone M, Iacobazzi RM, Annese C, Lopalco A, Lopedota AA, Franco M. TSPO Ligand-Methotrexate Prodrug Conjugates: Design, Synthesis, and Biological Evaluation. International Journal of Molecular Sciences. 2016; 17(6):967. https://doi.org/10.3390/ijms17060967
Chicago/Turabian StyleLaquintana, Valentino, Nunzio Denora, Annalisa Cutrignelli, Mara Perrone, Rosa Maria Iacobazzi, Cosimo Annese, Antonio Lopalco, Angela Assunta Lopedota, and Massimo Franco. 2016. "TSPO Ligand-Methotrexate Prodrug Conjugates: Design, Synthesis, and Biological Evaluation" International Journal of Molecular Sciences 17, no. 6: 967. https://doi.org/10.3390/ijms17060967
APA StyleLaquintana, V., Denora, N., Cutrignelli, A., Perrone, M., Iacobazzi, R. M., Annese, C., Lopalco, A., Lopedota, A. A., & Franco, M. (2016). TSPO Ligand-Methotrexate Prodrug Conjugates: Design, Synthesis, and Biological Evaluation. International Journal of Molecular Sciences, 17(6), 967. https://doi.org/10.3390/ijms17060967