Mechanisms Underlying Activation of α1-Adrenergic Receptor-Induced Trafficking of AQP5 in Rat Parotid Acinar Cells under Isotonic or Hypotonic Conditions
Abstract
:1. Introduction
2. Results
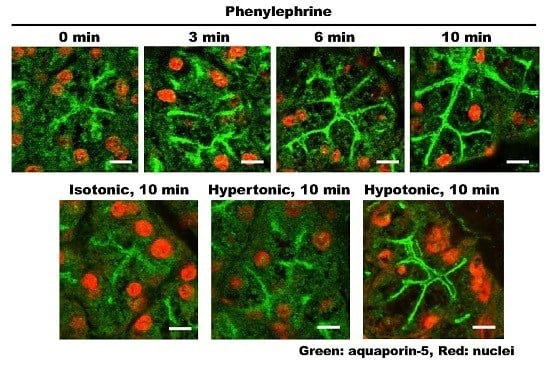
2.1. α1-AR Agonist-Induced Trafficking of AQP5 and Ganglioside GM1 to the APM and Lateral Plasma Membrane (LPM)
2.2. Prevention of α1-AR Agonist-Induced Trafficking of AQP5 and GM1 by Phentolamine
2.3. Purity of APM Isolated from Rat Parotid Gland
2.4. Effects of Antagonists of α1-AR Subtypes on the Phenylephrine-Induced Trafficking of AQP5
2.5. Prevention of Phenylephrine-Induced Trafficking of AQP5 by α1A-AR Antagonist
2.6. Effects of ODQ and KT5823 on AQP5 Levels in the APM of Phenylephrine-Stimulated Parotid Tissue
2.7. Effect of Differential Osmolality on AQP5 Trafficking to the APM in Parotid Tissues
2.8. Effects of Calcium Channel Blockers on Phenylephrine- and Hypotonicity-Induced Translocation of AQP5 in Rat Parotid Tissue
2.9. Phenylephrine-Induced AQP5 Translocation to the APM in Rat Parotid Tissues under Hypotonicity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Immunohistochemistry
4.4. Preparation of Rat Parotid Glands
4.4.1. Incubation of Rat Parotid Gland Slices
4.4.2. Preparation of APM from Parotid Gland Slices
4.5. Immunoblot Analysis
4.6. Osmolality
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| APM | Apical plasma membrane |
| AQP | Aquaporin |
| AR | Adrenergic receptor |
| BLM | Basolateral plasma membrane |
| BMY7378 | 8-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-8-azaspiro[4.5]decane-7,9-dione dihydrochloride |
| CaM | Calcium/calmodulin-dependent kinase |
| cGC | Soluble guanylate cyclase |
| cGMP | Cyclic guanosine monophosphate |
| γ-GT | γ-glutamyl transpeptidase |
| GM | Monosialotetrahexosylganglioside |
| HEK293 | Human Embryonic Kidney 293 cells |
| HEPESr | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| K+-NPPase | K+-stimulated p-nitrophenyl phosphatase |
| KRT buffer | Krebs-Ringer-Tris buffer |
| KT5823 | (9S,10R,12R)-2,3,9,10,11,12-Hexahydro-10-methoxy-2,9-dimethyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg: 3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, methyl ester |
| L765314 | [(2S)-4-(4-amino-6,7-dimethoxy-2-quinazolinyl)-2-[[(1,1-dimethylethyl)amino]carbonyl]-1-piperazinecarboxylic acid] |
| LPM | Lateral plasma membrane |
| LUTS | Lower urinary tract symptoms |
| mAChR | Muscarinic acetylcholine receptor |
| NOS | Nitric-oxide synthase |
| ODQ | 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one |
| Orai | Calcium release-activated calcium channel protein |
| PBS | Phosphate buffered saline |
| PE | Phenylephrine |
| PK | Protein kinase |
| RR | Ruthenium red |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SOCE | Store operated calcium entry |
| STIM | Stromal Interaction Molecule |
| TRPC | Transient receptor potential channel |
| TRPV | Transient receptor potential vanilloid |
References
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. 2007, 133, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, I.S. Ca2+ signaling and regulation of fluid secretion in salivary gland acinar cells. Cell Calcium 2014, 55, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Kwon, T.H.; Frokiaer, J.; Agre, P. Regulation and dysregulation of aquaporins in water balance disorders. J. Intern. Med. 2007, 261, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Kondo, S.; Hara, S.; Morishita, Y. The evolutionary aspects of aquaporin family. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R566–R576. [Google Scholar] [CrossRef] [PubMed]
- Raina, S.; Preston, G.M.; Guggino, W.B.; Agre, P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J. Biol. Chem. 1995, 270, 1908–1912. [Google Scholar] [PubMed]
- Krane, C.M.; Melvin, J.E.; Nguyen, H.V.; Richardson, L.; Towne, J.E.; Doetschman, T.; Menon, A.G. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J. Biol. Chem. 2001, 276, 23413–23420. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Nakamoto, T.; Jaramillo, Y.; Choi, S.; Catalan, M.A.; Melvin, J.E. Functional differences in the acinar cells of the murine major salivary glands. J. Dent. Res. 2015, 94, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Eguchi, T.; Skowronski, M.T.; Ishida, H. Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochem. Biophys. Res. Commun. 1998, 245, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Skowronski, M.T.; Inoue, N.; Ishida, H. α1-adrenoceptor-induced trafficking of aquaporin-5 to the apical plasma membrane of rat parotid cells. Biochem. Biophys. Res. Commun. 1999, 265, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Cho, G.; Yuan, Z.; Inoue, N.; Nakae, Y. Aquaporin-5 water channel in lipid rafts of rat parotid glands. Biochim. Biophys. Acta 2006, 1758, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.; Bragiel, A.M.; Wang, D.; Pieczonka, T.D.; Skowronski, M.T.; Shono, M.; Nielsen, S.; Ishikawa, Y. Activation of muscarinic receptors in rat parotid acinar cells induces AQP5 trafficking to nuclei and apical plasma membrane. Biochim. Biophys. Acta 2015, 1850, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Yuan, Z.; Inoue, N.; Skowronski, M.T.; Nakae, Y.; Shono, M.; Cho, G.; Yasui, M.; Agre, P.; Nielsen, S. Identification of AQP5 in lipid rafts and its translocation to apical membranes by activation of M3 mAChRs in interlobular ducts of rat parotid gland. Am. J. Physiol. Cell Physiol. 2005, 289, C1303–C1311. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Iwata, F.; Wang, D.; Muraguchi, M.; Ooga, K.; Ohmoto, Y.; Takai, M.; Cho, G.; Kang, J.; Shono, M.; et al. Identification of aquaporin-5 and lipid rafts in human resting saliva and their release into cevimeline-stimulated saliva. Biochim. Biophys. Acta 2009, 1790, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Miura, Y.; Harazono, A.; Kanai-Azuma, M.; Akimoto, Y.; Kawakami, H.; Yamaguchi, T.; Toda, T.; Endo, T.; Tsubuki, M.; et al. Proteomic analysis of two types of exosomes in human whole saliva. Biol. Pharm. Bull. 2011, 34, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Hieble, J.P.; Bylund, D.B.; Clarke, D.E.; Eikenburg, D.C.; Langer, S.Z.; Lefkowitz, R.J.; Minneman, K.P.; Ruffolo, R.R., Jr. International Union of Pharmacology. X. Recommendation for nomenclature of α 1-adrenoceptors: Consensus update. Pharmacol. Rev. 1995, 47, 267–270. [Google Scholar] [PubMed]
- Porter, J.E.; Dowd, F.J.; Abel, P.W. Atypical α-1 adrenergic receptors on the rat parotid gland acinar cell. J. Pharmacol. Exp. Ther. 1992, 263, 1062–1067. [Google Scholar] [PubMed]
- Rokosh, D.G.; Bailey, B.A.; Stewart, A.F.; Karns, L.R.; Long, C.S.; Simpson, P.C. Distribution of α1C-adrenergic receptor mRNA in adult rat tissues by RNase protection assay and comparison with α1B and α1D. Biochem. Biophys. Res. Commun. 1994, 200, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Goi, Y.; Tomiyama, Y.; Yokoyama, A.; Tatemichi, S.; Maruyama, K.; Kobayashi, M.; Yamaguchi, O. Effect of silodosin, a selective α1A-adrenoceptor antagonist, on voiding behavior and bladder blood flow in a rat model of bladder outlet obstruction. Eur. J. Pharmacol. 2015, 764, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, K.; Yoshida, M.; Homma, Y. Silodosin, a new α1A-adrenoceptor-selective antagonist for treating benign prostatic hyperplasia: Results of a phase III randomized, placebo-controlled, double-blind study in Japanese men. BJU Int. 2006, 98, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Hora, M.; Tatemichi, S.; Masuda, N.; Nakamura, S.; Yamagishi, R.; Kitazawa, M. KMD-3213, a uroselective and long-acting α1A-adrenoceptor antagonist, tested in a novel rat model. J. Pharmacol. Exp. Ther. 1999, 291, 81–91. [Google Scholar] [PubMed]
- Ozsoy, M.; Liatsikos, E.; Scheffbuch, N.; Kallidonis, P. Comparison of silodosin to tamsulosin for medical expulsive treatment of ureteral stones: A systematic review and meta-analysis. Urolithiasis 2016, in press. [Google Scholar]
- Noguchi, Y.; Ohtake, A.; Suzuki, M.; Sasamata, M. In vivo study on the effects of α1-adrenoceptor antagonists on intraurethral pressure in the prostatic urethra and intraluminal pressure in the vas deferens in male dogs. Eur. J. Pharmacol. 2008, 580, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Romeo, G.; Salerno, L.; Pittala, V.; Modica, M.N.; Siracusa, M.A.; Materia, L.; Buccioni, M.; Marucci, G.; Minneman, K.P. High affinity ligands and potent antagonists for the α1D-adrenergic receptor. Novel 3,8-disubstituted [1]benzothieno[3,2-d]pyrimidine derivatives. Eur. J. Med. Chem. 2014, 83, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Yanai-Inamura, H.; Ohtake, A.; Noguchi, Y.; Hatanaka, T.; Suzuki, M.; Ueshima, K.; Sato, S.; Sasamata, M. Effects of α1-adrenoceptor antagonists on phenylephrine-induced salivary secretion and intraurethral pressure elevation in anesthetized rats. Eur. J. Pharmacol. 2012, 679, 127–131. [Google Scholar] [CrossRef] [PubMed]
- McWhinney, C.; Wenham, D.; Kanwal, S.; Kalman, V.; Hansen, C.; Robishaw, J.D. Constitutively active mutants of the α1A- and the α1B-adrenergic receptor subtypes reveal coupling to different signaling pathways and physiological responses in rat cardiac myocytes. J. Biol. Chem. 2000, 275, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Bruchas, M.R.; Toews, M.L.; Bockman, C.S.; Abel, P.W. Characterization of the α1-adrenoceptor subtype activating extracellular signal-regulated kinase in submandibular gland acinar cells. Eur. J. Pharmacol. 2008, 578, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Conner, A.C.; Bill, R.M.; Conner, M.T. An emerging consensus on aquaporin translocation as a regulatory mechanism. Mol. Membr. Biol. 2013, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bandyopadhyay, B.C.; Nakamoto, T.; Singh, B.; Liedtke, W.; Melvin, J.E.; Ambudkar, I. A role for AQP5 in activation of TRPV4 by hypotonicity: Concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J. Biol. Chem. 2006, 281, 15485–15495. [Google Scholar] [CrossRef] [PubMed]
- Sidhaye, V.K.; Guler, A.D.; Schweitzer, K.S.; D’Alessio, F.; Caterina, M.J.; King, L.S. Transient receptor potential vanilloid 4 regulates aquaporin-5 abundance under hypotonic conditions. Proc. Natl. Acad. Sci. USA 2006, 103, 4747–4752. [Google Scholar] [CrossRef] [PubMed]
- Eckhard, A.; Dos Santos, A.; Liu, W.; Bassiouni, M.; Arnold, H.; Gleiser, C.; Hirt, B.; Harteneck, C.; Muller, M.; Rask-Andersen, H. Regulation of the perilymphatic-endolymphatic water shunt in the cochlea by membrane translocation of aquaporin-5. Pflug. Arch. 2015, 467, 2571–2588. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Oberg, F.; Sjohamn, J.; Hedfalk, K.; Bill, R.M.; Conner, A.C.; Conner, M.T.; Tornroth-Horsefield, S. Plasma membrane abundance of human aquaporin 5 is dynamically regulated by multiple pathways. PLoS ONE 2015, 10, e0143027. [Google Scholar] [CrossRef] [PubMed]
- Aure, M.H.; Roed, A.; Galtung, H.K. Intracellular Ca2+ responses and cell volume regulation upon cholinergic and purinergic stimulation in an immortalized salivary cell line. Eur. J. Oral. Sci. 2010, 118, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Romero-Calvo, I.; Ocon, B.; Martinez-Moya, P.; Suarez, M.; Zarzuelo, A.; Martinez-Augustin, O.; Sanchez de Media, F. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 2010, 401, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Gilda, J.E.; Gomes, A.V. The necessity of and strategies for improving confidence in the accuracy of western blots. Expert Rev. Proteom. 2014, 11, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Kizhatil, K.; Davis, J.Q.; Davis, L.; Hoffman, J.; Hogan, B.L.; Bennett, V. Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J. Biol. Chem. 2007, 282, 26552–26561. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Iida, H.; Ishida, H. The muscarinic acetylcholine receptor-stimulated increase in aquaporin-5 levels in the apical plasma membrane in rat parotid acinar cells is coupled with activation of nitric oxide/cGMP signal transduction. Mol. Pharmacol. 2002, 61, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.B.; Putney, J.W., Jr. Store-operated calcium channels. Physiol. Rev. 2005, 85, 757–810. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, T.; Yakata, A.; Izawa, T.; Suda, K.; Rubin, R.P.; Tsuboi, M. Protein kinase C-dependent diacylglycerol formation is mediated via Ca2+/calmodulin in parotid cells. Eur. J. Pharmacol. 1991, 207, 175–181. [Google Scholar] [CrossRef]
- Zimmermann, B. Calcium store depletion activates two distinct calcium entry pathways in secretory cells of the blowfly salivary gland. Cell Calcium 1998, 23, 53–63. [Google Scholar] [CrossRef]
- Alessandri-Haber, N.; Yeh, J.J.; Boyd, A.E.; Parada, C.A.; Chen, X.; Reichling, D.B.; Levine, J.D. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 2003, 39, 497–511. [Google Scholar] [CrossRef]
- Zhang, Y.; Catalan, M.A.; Melvin, J.E. TRPV4 activation in mouse submandibular gland modulates Ca2+ influx and salivation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1365–G1372. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, J.H.; Bundgaard, M. Quantitative estimation of the area of luminal and basolateral membranes of rat parotid acinar cells: Some physiological applications. Pflug. Arch. 1994, 429, 240–244. [Google Scholar] [CrossRef]
- Kawedia, J.D.; Nieman, M.L.; Boivin, G.P.; Melvin, J.E.; Kikuchi, K.; Hand, A.R.; Lorenz, J.N.; Menon, A.G. Interaction between transcellular and paracellular water transport pathways through Aquaporin 5 and the tight junction complex. Proc. Natl. Acad. Sci. USA 2007, 104, 3621–3626. [Google Scholar] [CrossRef] [PubMed]
- Docherty, J.R. Subtypes of functional α1-adrenoceptor. Cell. Mol. Life Sci. 2010, 67, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Drouin, C.; Darracq, L.; Trovero, F.; Blanc, G.; Glowinski, J.; Cotecchia, S.; Tassin, J.P. α1B-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J. Neurosci. 2002, 22, 2873–2884. [Google Scholar] [PubMed]
- Collette, K.M.; Zhou, X.D.; Amoth, H.M.; Lyons, M.J.; Papay, R.S.; Sens, D.A.; Perez, D.M.; Doze, V.A. Long-term α1B-adrenergic receptor activation shortens lifespan, while α1A-adrenergic receptor stimulation prolongs lifespan in association with decreased cancer incidence. Age 2014, 36, 9675. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, A.; Nasa, Y.; Koshimizu, T.; Shinoura, H.; Oshikawa, S.; Kawai, T.; Sunada, S.; Takeo, S.; Tsujimoto, G. The α1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J. Clin. Investig. 2002, 109, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Minneman, K.P. Recent progress in α1-adrenergic receptor research. Acta Pharmacol. Sin. 2005, 26, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Foote, J.; Glavind, K.; Kralidis, G.; Wyndaele, J.J. Treatment of overactive bladder in the older patient: Pooled analysis of three phase III studies of darifenacin, an M3 selective receptor antagonist. Eur. Urol. 2005, 48, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Ono, K.; Masuda, W.; Iida, T.; Hosokawa, R.; Inenaga, K. Differences in the Ca2+ response resulting from neurotransmitter stimulations of rat parotid acini and ducts. Auton. Neurosci. 2010, 154, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Sugiya, H.; Mitsui, Y.; Michikawa, H.; Fujita-Yoshigaki, J.; Hara-Yokoyama, M.; Hashimoto, S.; Furuyama, S. Ca2+-regulated nitric oxide generation in rabbit parotid acinar cells. Cell Calcium 2001, 30, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Rosignoli, F.; Perez Leiros, C. Activation of nitric oxide synthase through muscarinic receptors in rat parotid gland. Eur. J. Pharmacol. 2002, 439, 27–33. [Google Scholar] [CrossRef]
- Lucas, K.A.; Pitari, G.M.; Kazerounian, S.; Ruiz-Stewart, I.; Park, J.; Schulz, S.; Chepenik, K.P.; Waldman, S.A. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol. Rev. 2000, 52, 375–414. [Google Scholar] [PubMed]
- Kitchen, P.; Day, R.E.; Taylor, L.H.; Salman, M.M.; Bill, R.M.; Conner, M.T.; Conner, A.C. Identification and molecular mechanisms of the rapid tonicity-induced relocalization of the Aquaporin 4 channel. J. Biol. Chem. 2015, 290, 16873–16881. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Hartl, D.; Nolden, T.; Koppelstätter, A.; Klose, J.; Himmelbauer, H.; Zabel, C. Author information. Pronounced alterations of cellular metabolism and structure due to hyper- or hypo-osmosis. J. Proteome Res. 2008, 7, 3968–3983. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Watanabe, M.; Seto, M.; Hisatsune, A.; Miyata, T.; Isohama, Y. Nitric oxide decreases cell surface expression of aquaporin-5 and membrane water permeability in lung epithelial cells. Biochem. Biophys. Res. Commun. 2007, 354, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Soltoff, S.P.; Hedden, L. Isoproterenol and cAMP block ERK phosphorylation and enhance [Ca2+]i increases and oxygen consumption by muscarinic receptor stimulation in rat parotid and submandibular acinar cells. J. Biol. Chem. 2010, 285, 13337–13348. [Google Scholar] [CrossRef] [PubMed]
- Tojyo, Y.; Tanimura, A.; Nezu, A.; Matsumoto, Y. Activation of β-adrenoceptors does not cause any change in cytosolic Ca2+ distribution in rat parotid acinar cells. Eur. J. Pharmacol. 1998, 360, 73–79. [Google Scholar] [CrossRef]
- Hughes, A.R.; Takemura, H.; Putney, J.W., Jr. Does β-adrenoceptor activation stimulate Ca2+ mobilization and inositol trisphosphate formation in parotid acinar cells? Cell Calcium 1989, 10, 519–525. [Google Scholar] [CrossRef]
- Pani, B.; Singh, B.B. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium 2009, 45, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Pani, B.; Liu, X.; Bollimuntha, S.; Cheng, K.T.; Niesman, I.R.; Zheng, C.; Achen, V.R.; Patel, H.H.; Ambudkar, I.S.; Singh, B.B. Impairment of TRPC1-STIM1 channel assembly and AQP5 translocation compromise agonist-stimulated fluid secretion in mice lacking caveolin1. J. Cell Sci. 2013, 126, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Nashida, T.; Yoshie, S.; Imai, A.; Shimomura, H. Co-localization of rab4 with endocytosis-related proteins in the rat parotid glands. Arch. Histol. Cytol. 2003, 66, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Mottet, I.; Quast, M.J.; Dewitt, D.S.; Hillman, G.R.; Wei, J.; Uhrbrock, D.H.; Perez-Polo, J.R.; Kent, T.A. NG-nitro-L-arginine methyl ester modifies the input function measured by dynamic susceptibility contrast magnetic resonance imaging. J. Cereb. Blood Flow Metab. 1997, 17, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Eguchi, K.; Sakurai, H.; Ohmichi, Y.; Hashimoto, T.; Ohmichi, M.; Morimoto, A.; Yamaguchi, Y.; Ushida, T.; Iwase, S.; et al. Frequency components of systolic blood pressure variability reflect vasomotor and cardiac sympathetic functions in conscious rats. J. Physiol. Sci. 2011, 61, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Longbottom, D.; van Heyningen, S. The activation of rabbit intestinal adenylate cyclase by cholera toxin. Biochim. Biophys. Acta 1989, 1014, 289–297. [Google Scholar] [CrossRef]
- Meister, A.; Tate, S.S.; Griffith, O.W. γ-Glutamyl transpeptidase. Methods Enzymol. 1981, 77, 237–253. [Google Scholar] [PubMed]
- Alves, R.; Barbosa de Carvalho, J.G.; Benedito, M.A. High and low rearing subgroups of rats selected in the open field differ in the activity of K+-stimulated p-nitrophenylphosphatase in the hippocampus. Brain Res. 2005, 1058, 178–182. [Google Scholar] [CrossRef] [PubMed]









© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bragiel, A.M.; Wang, D.; Pieczonka, T.D.; Shono, M.; Ishikawa, Y. Mechanisms Underlying Activation of α1-Adrenergic Receptor-Induced Trafficking of AQP5 in Rat Parotid Acinar Cells under Isotonic or Hypotonic Conditions. Int. J. Mol. Sci. 2016, 17, 1022. https://doi.org/10.3390/ijms17071022
Bragiel AM, Wang D, Pieczonka TD, Shono M, Ishikawa Y. Mechanisms Underlying Activation of α1-Adrenergic Receptor-Induced Trafficking of AQP5 in Rat Parotid Acinar Cells under Isotonic or Hypotonic Conditions. International Journal of Molecular Sciences. 2016; 17(7):1022. https://doi.org/10.3390/ijms17071022
Chicago/Turabian StyleBragiel, Aneta M., Di Wang, Tomasz D. Pieczonka, Masayuki Shono, and Yasuko Ishikawa. 2016. "Mechanisms Underlying Activation of α1-Adrenergic Receptor-Induced Trafficking of AQP5 in Rat Parotid Acinar Cells under Isotonic or Hypotonic Conditions" International Journal of Molecular Sciences 17, no. 7: 1022. https://doi.org/10.3390/ijms17071022
APA StyleBragiel, A. M., Wang, D., Pieczonka, T. D., Shono, M., & Ishikawa, Y. (2016). Mechanisms Underlying Activation of α1-Adrenergic Receptor-Induced Trafficking of AQP5 in Rat Parotid Acinar Cells under Isotonic or Hypotonic Conditions. International Journal of Molecular Sciences, 17(7), 1022. https://doi.org/10.3390/ijms17071022