Hepatocyte and Sertoli Cell Aquaporins, Recent Advances and Research Trends
Abstract
:1. Introduction
2. Hepatocyte Aquaporins: Physiology, Pathophysiology and Potential Relevance as Drug Targets
2.1. Expression and Subcellular Localization of Hepatocyte Aquaporins
2.2. Involvement in Metabolic Homeostasis and Energy Balance
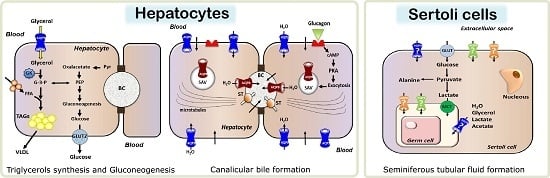
2.2.1. Aquaporin 9 (AQP9)-Mediated Glycerol Import in Gluconeogenesis and Triacylglycerols Synthesis
2.2.2. Aquaporin 8 (AQP8) in Mitochondrial Ammonia Detoxification
2.2.3. Aquaporin 8 (AQP8) in the Hepatic Metabolism of Glycogen
2.3. Roles in Primary Bile Formation and Secretion
2.4. Mitochondrial Aquaporin 8 (AQP8) and Reactive Oxygen Species (ROS) Release
2.5. Hepatocyte Aquaporins in Fatty Liver Disease, Obesity and Diabetes Mellitus
2.6. Relevance in Bile Secretory Disorders
2.7. Involvement of Hepatocyte Aquaporins in Other Diseases
2.8. Potential Pharmacological and Gene Transfer Applications
3. Aquaporins in Sertoli Cells: Expression, Physiology and Potential Roles in Male Reproductive Potential
3.1. The Sertoli Cell: A Brief Overview
3.2. Testicular Metabolic Cooperation between Sertoli: Germ Cells: A Selective Process of Nutrients and Fluids
3.3. Expression and Subcellular Localization of Aquaporins in Sertoli Cells
3.4. Aquaporins Functionality in Testis and Their Possible Relevance for Sertoli Cell Metabolism
4. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agre, P. Aquaporin water channels (Nobel Lecture). Angew. Chem. Int. Ed. 2004, 43, 4278–4290. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Portincasa, P. The power of Science Diplomacy, a lesson from the Nobel Laureate Peter Agre. Eur. J. Clin. Investig. 2016, 46, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Delporte, C.; Marinelli, R.A. Hepatobiliary, salivary glands and pancreas aquaporins in health and disease. In Aquaporins in Health and Disease: New Molecular Targets for Drug Discovery; Soveral, G., Casini, A., Nielsen, S., Eds.; Chapter 9; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2015; pp. 193–196. [Google Scholar]
- Marinelli, R.A.; Lehmann, G.L.; Soria, L.R.; Marchissio, M.J. Hepatocyte aquaporins in bile formation and cholestasis. Front. Biosci. (Landmark Ed.) 2011, 17, 2642–2652. [Google Scholar] [CrossRef]
- Calamita, G.; Mazzone, A.; Bizzoca, A.; Cavalier, A.; Cassano, G.; Thomas, D.; Svelto, M. Expression and immunolocalization of the aquaporin-8 water channel in rat gastrointestinal tract. Eur. J. Cell Biol. 2001, 80, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Elkjaer, M.L.; Nejsum, L.N.; Gresz, V.; Kwon, T.H.; Jensen, U.B.; Frøkiaer, J.; Nielsen, S. Immunolocalization of aquaporin-8 in rat kidney, gastrointestinal tract, testis, and airways. Am. J. Physiol. 2001, 281, F1047–F1057. [Google Scholar] [CrossRef]
- García, F.; Kierbel, A.; Larocca, M.C.; Gradilone, S.A.; Splinter, P.; LaRusso, N.F.; Marinelli, R.A. The water channel aquaporin-8 is mainly intracellular in rat hepatocytes, and its plasma membrane insertion is stimulated by cyclic AMP. J. Biol. Chem. 2001, 276, 12147–12152. [Google Scholar] [CrossRef] [PubMed]
- Ferri, D.; Mazzone, A.; Liquori, G.E.; Cassano, G.; Svelto, M.; Calamita, G. Ontogeny, distribution, and possible functional implications of an unusual aquaporin, AQP8, in mouse liver. Hepatology 2003, 38, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Ferri, D.; Gena, P.; Liquori, G.E.; Cavalier, A.; Thomas, D.; Svelto, M. The inner mitochondrial membrane has aquaporin-8 water channels and is highly permeable to water. J. Biol. Chem. 2005, 280, 17149–17153. [Google Scholar] [CrossRef] [PubMed]
- Gradilone, S.A.; García, F.; Huebert, R.C.; Tietz, P.S.; Larocca, M.C.; Kierbel, A.; Carreras, F.I.; LaRusso, N.F.; Marinelli, R.A. Glucagon induces the plasma membrane insertion of functional aquaporin-8 water channels in isolated rat hepatocytes. Hepatology 2003, 37, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Tietz, P.; Jefferson, J.; Pagano, R.; Larusso, N.F. Membrane microdomains in hepatocytes: Potential target areas for proteins involved in canalicular bile secretion. J. Lipid Res. 2005, 46, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, A.; Tietz, P.; Jefferson, J.; Pagano, R.; LaRusso, N.F. Isolation and characterization of lipid microdomains from apical and basolateral plasma membranes of rat hepatocytes. Hepatology 2006, 43, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Gradilone, S.A.; Carreras, F.I.; Lehmann, G.L.; Marinelli, R.A. Phosphoinositide 3-kinase is involved in the glucagon-induced translocation of aquaporin-8 to hepatocyte plasma membrane. Biol. Cell 2005, 97, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Soria, L.R.; Gradilone, S.A.; Larocca, M.C.; Marinelli, R.A. Glucagon induces the gene expression of aquaporin-8 but not that of aquaporin-9 water channels in the rat hepatocyte. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1274–R1281. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Calamita, G. Water channel proteins in bile formation and flow in health and disease: When immiscible becomes miscible. Mol. Asp. Med. 2012, 33, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Gena, P.; Meleleo, D.; Ferri, D.; Svelto, M. Water permeability of rat liver mitochondria: A biophysical study. Biochim. Biophys. Acta 2006, 1758, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhao, D.; Verkman, A.S. Evidence against functionally significant aquaporin expression in mitochondria. J. Biol. Chem. 2006, 281, 16202–16206. [Google Scholar] [CrossRef] [PubMed]
- Gena, P.; Fanelli, E.; Brenner, C.; Svelto, M.; Calamita, G. News and views on mitochondrial water transport. Front. Biosci. 2009, 1, 352–361. [Google Scholar] [CrossRef]
- Saparov, S.M.; Liu, K.; Agre, P.; Pohl, P. Fast and selective ammonia transport by aquaporin-8. J. Biol. Chem. 2007, 282, 5296–5301. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Moller, A.L.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Soria, L.R.; Marrone, J.; Calamita, G.; Marinelli, R.A. Ammonia detoxification via ureagenesis in rat hepatocytes involves mitochondrial aquaporin-8 channels. Hepatology 2013, 57, 2061–2071. [Google Scholar] [CrossRef] [PubMed]
- Marchissio, M.J.; Francés, D.E.A.; Carnovale, C.E.; Marinelli, R.A. Mitochondrial aquaporin-8 knockdown in human hepatoma HepG2 cells causes ROS-induced mitochondrial depolarization and loss of viability. Toxicol. Appl. Pharmacol. 2012, 264, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, R.A.; Marchissio, M.J. Mitochondrial aquaporin-8: A functional peroxiporin? Antioxid. Redox Signal. 2013, 19, 896. [Google Scholar] [CrossRef] [PubMed]
- Elkjaer, M.L.; Vajda, Z.; Nejsum, L.N.; Kwon, T.; Jensen, U.B.; Amiry-Moghaddam, M.; Frøkiaer, J.; Nielsen, S. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem. Biophys. Res. Commun. 2000, 276, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Carbrey, J.M.; Gorelick-Feldman, D.A.; Kozono, D.; Praetorius, J.; Nielsen, S.; Agre, P. Aquaglyceroporin AQP9: Solute permeation and metabolic control of expression in liver. Proc. Natl. Acad. Sci. USA 2003, 100, 2945–2950. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Marinelli, R.A.; Tesse, A.; Frühbeck, G.; Calamita, G. Sexual Dimorphism of Adipose and Hepatic Aquaglyceroporins in Health and Metabolic Disorders. Front. Endocrinol. (Lausanne) 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nicchia, G.P.; Frigeri, A.; Nico, B.; Ribatti, D.; Svelto, M. Tissue distribution and membrane localization of aquaporin-9 water channel: Evidence for sex-linked differences in liver. J. Histochem. Cytochem. 2001, 49, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Lebeck, J.; Gena, P.; O’Neill, H.; Skowronski, M.T.; Lund, S.; Calamita, G.; Praetorius, J. Estrogen prevents increased hepatic aquaporin-9 expression and glycerol uptake during starvation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G365–G374. [Google Scholar] [CrossRef] [PubMed]
- Jelen, S.; Wacker, S.; Aponte-Santamaría, C.; Skott, M.; Rojek, A.; Johanson, U.; Kjellbom, P.; Nielsen, S.; de Groot, B.L.; Rützler, M. Aquaporin-9 protein is the primary route of hepatocyte glycerol uptake for glycerol gluconeogenesis in mice. J. Biol. Chem. 2011, 286, 44319–44325. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Gena, P.; Ferri, D.; Rosito, A.; Rojek, A.; Marinelli, R.A.; Frühbeck, G.; Svelto, M. Biophysical assessment of aquaporin-9 as principal facilitative pathway in mouse liver import of glucogenetic glycerol. Biol. Cell 2012, 104, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Jelen, S.; Gena, P.; Lebeck, J.; Rojek, A.; Praetorius, J.; Frøkiaer, J.; Fenton, R.A.; Nielsen, S.; Calamita, G.; Rützler, M. Aquaporin-9 and urea transporter-A gene deletions affect urea transmembrane passage in murine hepatocytes. Am. J. Physiol. 2012, 303, G1279–G1287. [Google Scholar] [CrossRef] [PubMed]
- Carbrey, J.M.; Song, L.; Zhou, Y.; Yoshinaga, M.; Rojek, A.; Wang, Y.; Liu, Y.; Lujan, H.L.; DiCarlo, S.E.; Nielsen, S.; et al. Reduced arsenic clearance and increased toxicity in aquaglyceroporin-9-null mice. Proc. Natl. Acad. Sci. USA 2009, 106, 15956–15960. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Moniaga, C.S.; Nielsen, S.; Hara-Chikuma, M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016, 471, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Tanaka, Y.; Morishita, Y. The role of mammalian superaquaporins inside the cell. Biochim. Biophys. Acta 2014, 1840, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Reshef, L.; Olswang, Y.; Cassuto, H.; Blum, B.; Croniger, C.M.; Kalhan, S.C.; Tilghman, S.M.; Hanson, R.W. Glyceroneogenesis and the triglyceride/fatty acid cycle. J. Biol. Chem. 2003, 278, 30413–30416. [Google Scholar] [CrossRef] [PubMed]
- D’Abbicco, M.; Del Buono, N.; Gena, P.; Berardi, M.; Calamita, G.; Lopez, L. A model for the hepatic glucose metabolism based on Hill and step functions. J. Comput. Appl. Math. 2016, 292, 746–759. [Google Scholar] [CrossRef]
- Patsouris, D.; Mandard, S.; Voshol, P.J.; Escher, P.; Tan, N.S.; Havekes, L.M.; Koenig, W.; März, W.; Tafuri, S.; Wahli, W.; et al. PPARα governs glycerol metabolism. J. Clin. Investig. 2004, 114, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Lebeck, J.; Cheema, M.U.; Skowronski, M.T.; Nielsen, S.; Praetorius, J. Hepatic AQP9 expression in male rats is reduced in response to PPARα agonist treatment. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G198–G205. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.M.; Skowronski, M.T.; Fuchtbauer, E.M.; Füchtbauer, A.C.; Fenton, R.A.; Agre, P.; Frøkiaer, J.; Nielsen, S. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc. Natl. Acad. Sci. USA 2007, 104, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, H.; Shimomura, I.; Kishida, K.; Kondo, H.; Furuyama, N.; Nishizawa, H.; Maeda, N.; Matsuda, M.; Nagaretani, H.; Kihara, S.; et al. Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes 2002, 51, 2915–2921. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Gena, P.; Méndez-Gimenez, L.; Rosito, A.; Valentí, V.; Rotellar, F.; Sola, I.; Moncada, R.; Silva, C.; Svelto, M.; et al. Reduced hepatic aquaporin-9 and glycerol permeability are related to insulin resistance in non-alcoholic fatty liver disease. Int. J. Obes. 2014, 38, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Catalan, V.; Gomez-Ambrosi, J.; García-Navarro, S.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Burrell, M.A.; et al. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J. Clin. Endocrinol. Metab. 2011, 96, E586–E597. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Iguchi, K.; Usui, S.; Hirano, K. AMP-activated protein kinase modulates the gene expression of aquaporin 9 via forkhead box a2. Arch. Biochem. Biophys. 2011, 515, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Ceperuelo-Mallafre, V.; Lecube, A.; Hernandez, C.; Chacon, M.R.; Fort, J.M.; Gallart, L.; Baena-Fustegueras, J.A.; Simó, R.; Vendrell, J. Gene expression of paired abdominal adipose AQP7 and liver AQP9 in patients with morbid obesity: relationship with glucose abnormalities. Metabolism 2009, 58, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Giménez, L.; Rodríguez, A.; Balaguer, I.; Frühbeck, G. Aquaglyceroporins and caveolins in energy and metabolic homeostasis. Mol. Cell. Endocrinol. 2014, 397, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Catalán, V.; Gómez-Ambrosi, J.; Frühbeck, G. Aquaglyceroporins serve as metabolic gateways in adiposity and insulin resistance control. Cell Cycle 2011, 10, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Lebeck, J. Metabolic impact of the glycerol channels AQP7 and AQP9 in adipose tissue and liver. J. Mol. Endocrinol. 2014, 52, R165–R178. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.M.; Jahn, T.P.; Møller, A.L.; Schjoerring, J.K.; Ferri, D.; Klaerke, D.A.; Zeuthen, T. NH3 and NH4 permeability in aquaporin-expressing Xenopus oocytes. Pflugers Arch. 2005, 450, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Jahn, T.P.; Møller, A.L.B.; Zeuthen, T.; Holm, L.M.; Klaerke, D.A.; Mohsin, B.; Kühlbrandt, W.; Schjoerring, J.K. Aquaporin homologues in plants and mammals transport ammonia. FEBS Lett. 2004, 574, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Nagase, H.; Huang, C.G.; Calamita, G.; Agre, P. Purification and functional characterization of aquaporin-8. Biol. Cell 2006, 98, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhao, D.; Solenov, E.; Verkman, A.S. Evidence from knockout mice against physiologically significant aquaporin 8-facilitated ammonia transport. Am. J. Physiol. 2006, 291, C417–C423. [Google Scholar] [CrossRef] [PubMed]
- Soria, L.R.; Fanelli, E.; Altamura, N.; Svelto, M.; Marinelli, R.A.; Calamita, G. Aquaporin-8-facilitated mitochondrial ammonia transport. Biochem. Biophys. Res. Commun. 2010, 393, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Moreno, M.; Ferri, D.; Silvestri, E.; Roberti, P.; Schiavo, L.; Gena, P.; Svelto, M.; Goglia, F. Triiodothyronine modulates the expression of aquaporin-8 in rat liver mitochondria. J. Endocrinol. 2007, 192, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Soria, L.R.; Marrone, J.; Molinas, S.M.; Lehmann, G.L.; Calamita, G.; Marinelli, R.A. Lipopolysaccharide impairs hepatocyte ureagenesis from ammonia: Involvement of mitochondrial aquaporin-8. FEBS Lett. 2014, 588, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.L. Bile formation and secretion. Compr. Physiol. 2013, 3, 1035–1078. [Google Scholar] [PubMed]
- Huebert, R.C.; Splinter, P.L.; García, F.; Marinelli, R.A.; LaRusso, N.F. Expression and localization of aquaporin water channels in rat hepatocytes. Evidence for a role in canalicular bile secretion. J. Biol. Chem. 2002, 277, 22710–22717. [Google Scholar] [CrossRef] [PubMed]
- Larocca, M.C.; Soria, L.R.; Espelt, M.V.; Lehmann, G.L.; Marinelli, R.A. Knockdown of hepatocyte aquaporin-8 by RNA interference induces defective bile canalicular water transport. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G93–G100. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, R.A.; Tietz, P.S.; Caride, A.J.; Huang, B.Q.; LaRusso, N.F. Water transporting properties of hepatocyte basolateral and canalicular plasma membrane domains. J. Biol. Chem. 2003, 278, 43157–43162. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.R.; Soria, L.R.; Ventimiglia, M.S.; Najenson, A.C.; Di María, A.; Dabas, P.; Fellet, A.; Marinelli, R.A.; Vatta, M.S.; Bianciotti, L.G. Endothelin-1 and -3 induce choleresis in the rat through ETB receptors coupled to nitric oxide and vagovagal reflexes. Clin. Sci. (Lond.) 2013, 125, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Rigoulet, M.; Yoboue, E.D.; Devin, A. Mitochondrial ROS generation and its regulation: Mechanisms involved in H2O2 signaling. Antioxid. Redox Signal. 2011, 14, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signalling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Marchissio, M.J.; Francés, D.E.A.; Carnovale, C.E.; Marinelli, R.A. Evidence for necrosis, but not apoptosis, in human hepatoma cells with knockdown of mitochondrial aquaporin-8. Apoptosis 2014, 19, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J.; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterologyh. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012, 142, 1592–1609. [Google Scholar] [PubMed]
- Tiniakos, D.G.; Vos, M.B.; Brunt, E.M. Nonalcoholic fatty liver disease: Pathology and pathogenesis. Annu. Rev. Pathol. 2010, 5, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Gena, P.; Mastrodonato, M.; Portincasa, P.; Fanelli, E.; Mentino, D.; Rodríguez, A.; Marinelli, R.A.; Brenner, C.; Frühbeck, G.; Svelto, M.; et al. Liver glycerol permeability and aquaporin-9 are dysregulated in a murine model of non-alcoholic fatty liver disease. PLoS ONE 2013, 8, e78139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portois, L.; Zhang, Y.; Ladriere, L.; Perret, J.; Louchami, K.; Gaspard, N.; Hupkens, E.; Bolaky, N.; Delforge, V.; Beauwens, R.; et al. Perturbation of glycerol metabolism in hepatocytes from n3-PUFA-depleted rats. Int. J. Mol. Med. 2012, 29, 1121–1126. [Google Scholar] [PubMed]
- Cai, C.; Wang, C.; Ji, W.; Liu, B.; Kang, Y.; Hu, Z.; Jiang, Z. Knockdown of hepatic aquaglyceroporin-9 alleviates high fat diet-induced non-alcoholic fatty liver disease in rats. Int. Immunopharmacol. 2013, 15, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Hirako, S.; Wakayama, Y.; Kim, H.; Iizuka, Y.; Matsumoto, A.; Wada, N.; Kimura, A.; Okabe, M.; Sakagami, J.; Suzuki, M.; et al. The relationship between aquaglyceroporin expression and development of fatty liver in diet-induced obesity and ob/ob mice. Obes. Res. Clin. Pract. 2015, S1871–403X, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Boyer, J.L. Molecular alterations in hepatocyte transport mechanisms in acquired cholestatic liver disorders. Semin. Liver Dis. 2000, 20, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Carreras, F.I.; Gradilone, S.A.; Mazzone, A.; García, F.; Huang, B.Q.; Ochoa, J.E.; Tietz, P.S.; Larusso, N.F.; Calamita, G.; Marinelli, R.A. Rat hepatocyte aquaporin-8 water channels are down-regulated in extrahepatic cholestasis. Hepatology 2003, 37, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Carreras, F.I.; Lehmann, G.L.; Ferri, D.; Tioni, M.F.; Calamita, G.; Marinelli, R.A. Defective hepatocyte aquaporin-8 expression and reduced canalicular membrane water permeability in estrogen-induced cholestasis. Am. J. Physiol. 2007, 292, G905–G912. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, G.L.; Carreras, F.I.; Soria, L.R.; Gradilone, S.A.; Marinelli, R.A. LPS induces the TNF-α-mediated downregulation of rat liver aquaporin-8: Role in sepsis-associated cholestasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G567–G575. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, G.L.; Marinelli, R.A. Peritoneal sepsis downregulates liver expression of Aquaporin-8: A water channel involved in bile secretion. Liver Int. 2009, 29, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Ferri, D.; Gena, P.; Carreras, F.I.; Liquori, G.E.; Portincasa, P.; Marinelli, R.A.; Svelto, M. Altered Expression and Distribution of Aquaporin-9 in the Liver of Rat with Obstructive Extrahepatic Cholestasis. Am. J. Physiol. 2008, 295, G682–G690. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; O’Connell, B.C.; He, X.; Lancaster, H.E.; O’Connell, A.C.; Agre, P.; Baum, B.J. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc. Natl. Acad. Sci. USA 1997, 94, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.J.; Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Goldsmith, C.M.; Burbelo, P.D.; Citrin, D.E.; Mitchell, J.B.; et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc. Natl. Acad. Sci. USA 2012, 109, 19403–19407. [Google Scholar] [CrossRef] [PubMed]
- Marrone, J.; Lehmann, G.L.; Soria, L.R.; Pellegrino, J.M.; Molinas, S.; Marinelli, R.A. Adenoviral transfer of human aquaporin-1 gene to rat liver improves bile flow in estrogen-induced cholestasis. Gene Ther. 2014, 21, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Marrone, J.; Soria, L.R.; Danielli, M.; Lehmann, G.L.; Larocca, M.C.; Marinelli, R.A. Hepatic gene transfer of human aquaporin-1 improves bile salt secretory failure in rats with estrogen-induced cholestasis. Hepatology 2016. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Sakube, Y.; Sasaki, S.; Ishibashi, K. Molecular mechanisms and drug development in aquaporin water channel diseases: Aquaporin superfamily (superaquaporins): Expansion of aquaporins restricted to multicellular organisms. J. Pharmacol. Sci. 2004, 96, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.; Füchtbauer, E.M.; Füchtbauer, A.; Jelen, S.; Malmendal, A.; Fenton, R.A.; Nielsen, S. Liver-specific Aquaporin 11 knockout mice show rapid vacuolization of the rough endoplasmic reticulum in periportal hepatocytes after amino acid feeding. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G501–G515. [Google Scholar] [CrossRef] [PubMed]
- Padma, S.; Smeltz, A.M.; Banks, P.M.; Iannitti, D.A.; McKillop, I.H. Altered aquaporin 9 expression and localization in human hepatocellular carcinoma. HPB (Oxf.) 2009, 11, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Lindskog, C.; Asplund, A.; Catrina, A.; Nielsen, S.; Rützler, M. A systematic characterization of aquaporin-9 expression in human normal and pathological tissues. J. Histochem. Cytochem. 2016, 64, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.G.; Li, C.F.; Liu, M.; Chen, X.F.; Shuai, K.; Kong, X.; Lv, L.; Mei, Z.C. Aquaporin 9 has is down-regulated in hepatocellular carcinoma and its over-expression suppresses hepatoma cell invasion through inhibiting epithelial-to-mesenchymal transition. Cancer Lett. 2016, 378, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Beitz, E.; Golldack, A.; Rothert, M.; von Bülow, J. Challenges and achievements in the therapeutic modulation of aquaporin functionality. Pharmacol. Ther. 2015, 155, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Wacker, S.J.; Aponte-Santamaría, C.; Kjellbom, P.; Nielsen, S.; de Groot, B.L.; Rützler, M. The identification of novel, high affinity AQP9 inhibitors in an intracellular binding site. Mol. Membr. Biol. 2013, 30, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Alves, M.G. The Sertoli cell at a glance. In Sertoli Cell Metabolism and Spermatogenesis; Owen, M., Ed.; Springer: London, UK, 2015; pp. 3–13. [Google Scholar]
- Mruk, D.D.; Cheng, C.Y. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 2004, 25, 747–806. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.D.; Bernardino, R.L.; Neuhaus-Oliveira, A.; Sousa, M.; Sá, R.; Alves, M.G.; Oliveira, P.F. Physiology of Na+/H+ exchangers in the male reproductive tract: Relevance for male fertility. Biol. Reprod. 2014, 91, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, R.L.; Jesus, T.T.; Martins, A.D.; Sousa, M.; Barros, A.; Cavaco, J.E.; Socorro, S.; Alves, M.G.; Oliveira, P.F. Molecular basis of bicarbonate membrane transport in the male reproductive tract. Curr. Med. Chem. 2013, 20, 4037–4049. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Meneses, M.J.; Silva, B.M.; Sousa, M.; Alves, M.G.; Oliveira, P.F. New insights on hormones and factors that modulate Sertoli cell metabolism. Histol. Histopathol. 2016, 31, 499–513. [Google Scholar] [PubMed]
- Alves, M.G.; Rato, L.; Carvalho, R.A.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell. Mol. Life Sci. 2013, 70, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Sousa, M.; Barros, A.; Moura, T.; da Costa, A.R. Membrane transporters and cytoplasmatic pH regulation on bovine Sertoli cells. J. Membr. Biol. 2009, 227, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Sousa, M.; Barros, A.; Moura, T.; da Costa, A.R. Intracellular pH regulation in human Sertoli cells: Role of membrane transporters. Reproduction 2009, 137, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Socorro, S.; Cavaco, J.E.; Oliveira, P.F. Tubular fluid secretion in the seminiferous epithelium: Ion transporters and aquaporins in Sertoli cells. J. Membr. Biol. 2010, 236, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, R.L.; Costa, A.R.; Martins, A.D.; Silva, J.; Barros, A.; Sousa, M.; Sá, R.; Alves, M.G.; Oliveira, P.F. Estradiol modulates Na+-dependent HCO3− transporters altering intracellular pH and ion transport in human Sertoli cells: A role on male fertility? Biol. Cell 2016. [Google Scholar] [CrossRef] [PubMed]
- Setchell, B. The functional significance of the blood-testis barrier. J. Androl. 1980, 1, 3–10. [Google Scholar] [CrossRef]
- Robinson, R.; Fritz, I.B. Metabolism of glucose by Sertoli cells in culture. Biol. Reprod. 1981, 24, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Boussouar, F.; Benahmed, M. Lactate and energy metabolism in male germ cells. Trends Endocrinol. Metab. 2004, 15, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Erkkilä, K.; Aito, H.; Aalto, K.; Pentikäinen, V.; Dunkel, L. Lactate inhibits germ cell apoptosis in the human testis. Mol. Hum. Reprod. 2002, 8, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Martins, A.D.; Moreira, A.C.; Cheng, C.Y.; Alves, M.G. The warburg effect revisited—Lesson from the Sertoli cell. Med. Res. Rev. 2015, 35, 126–151. [Google Scholar] [CrossRef] [PubMed]
- Grootegoed, J.; Oonk, R.; Jansen, R.; van der Molen, H. Metabolism of radiolabelled energy-yielding substrates by rat Sertoli cells. J. Reprod. Fertil. 1986, 77, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Carosa, E.; Radico, C.; Giansante, N.; Rossi, S.; D’Adamo, F.; di Stasi, S.M.; Lenzi, A.; Jannini, E.A. Ontogenetic profile and thyroid hormone regulation of type-1 and type-8 glucose transporters in rat Sertoli cells. Int. J. Androl. 2005, 28, 99–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokk, K.; Veräjänkorva, E.; Wu, X.; Tapfer, H.; Poldoja, E.; Pöllänen, P. Immunohistochemical detection of glucose transporters class i subfamily in the mouse, rat and human testis. Medicina 2003, 40, 156–160. [Google Scholar]
- Oliveira, P.F.; Alves, M.G.; Rato, L.; Laurentino, S.; Silva, J.; Sa, R.; Barros, A.; Sousa, M.; Carvalho, R.A.; Cavaco, J.E. Effect of insulin deprivation on metabolism and metabolism-associated gene transcript levels of in vitro cultured human Sertoli cells. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Alves, M.G.; Rato, L.; Silva, J.; Sa, R.; Barros, A.; Sousa, M.; Carvalho, R.A.; Cavaco, J.E.; Socorro, S. Influence of 5α-dihydrotestosterone and 17β-estradiol on human Sertoli cells metabolism. Int. J. Androl. 2011, 34, e612–e620. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.G.; Socorro, S.; Silva, J.; Barros, A.; Sousa, M.; Cavaco, J.E.; Oliveira, P.F. In vitro cultured human Sertoli cells secrete high amounts of acetate that is stimulated by 17β-estradiol and suppressed by insulin deprivation. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, M.; Waki, A.; Yonekura, Y.; Sadato, N.; Murata, T.; Omata, N.; Takahashi, N.; Welch, M.J.; Fujibayashi, Y. Characterization of acetate metabolism in tumor cells in relation to cell proliferation: Acetate metabolism in tumor cells. Nucl. Med. Biol. 2001, 28, 117–122. [Google Scholar] [CrossRef]
- Alves, M.G.; Martins, A.D.; Rato, L.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Molecular mechanisms beyond glucose transport in diabetes-related male infertility. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, M.; Gupta, G.; Setty, B. Changes in carbohydrate metabolism of testicular germ cells during meiosis in the rat. Eur. J. Endocrinol. 1998, 138, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Jutte, N.H.; Grootegoed, J.; Rommerts, F.; van der Molen, H. Exogenous lactate is essential for metabolic activities in isolated rat spermatocytes and spermatids. J. Reprod. Fertil. 1981, 62, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Wenger, R.H.; Katschinski, D.M. The hypoxic testis and post-meiotic expression of pas domain proteins. Semin. Cell Dev. Biol. 2005, 16, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, L.D.; Bartke, A.; Goh, J.C. Postnatal development of the Sertoli cell barrier, tubular lumen, and cytoskeleton of Sertoli and myoid cells in the rat, and their relationship to tubular fluid secretion and flow. Am. J. Anat. 1989, 184, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-F.; He, R.-H.; Sun, C.-C.; Zhang, Y.; Meng, Q.-X.; Ma, Y.-Y. Function of aquaporins in female and male reproductive systems. Hum. Reprod. Update 2006, 12, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Sohara, E.; Uchida, S.; Sasaki, S. Function of aquaporin-7 in the kidney and the male reproductive system. In Aquaporins; Beitz, E., Ed.; Springer: Berlin, Germany, 2009; Volume 190, pp. 219–231. [Google Scholar]
- Badran, H.H.; Hermo, L.S. Expression and regulation of aquaporins 1, 8, and 9 in the testis, efferent ducts, and epididymis of adult rats and during postnatal development. J. Androl. 2002, 23, 358–373. [Google Scholar] [PubMed]
- Hermo, L.; Krzeczunowicz, D.; Ruz, R. Cell specificity of aquaporins 0, 3, and 10 expressed in the testis, efferent ducts, and epididymis of adult rats. J. Androl. 2004, 25, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Callies, C.; Tuttelmann, F.; Kliesch, S.; Cooper, T.G. Aquaporins in the human testis and spermatozoa—Identification, involvement in sperm volume regulation and clinical relevance. Int. J. Androl. 2010, 33, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Sprando, R.L.; Russell, L.D. Comparative study of cytoplasmic elimination in spermatids of selected mammalian species. Am. J. Anat. 1987, 178, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Hermo, L.; Smith, C.E. Thirsty business: Cell, region, and membrane specificity of aquaporins in the testis, efferent ducts, and epididymis and factors regulating their expression. J. Androl. 2011, 32, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.G.; Sá, R.; Jesus, T.T.; Sousa, M.; Oliveira, P.F. CFTR regulation of Aquaporin-mediated water transport: A target in male fertility. Curr. Drug Targets 2015, 16, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Jesus, T.T.; Bernardino, R.L.; Martins, A.D.; Sá, R.; Sousa, M.; Alves, M.G.; Oliveira, P.F. Aquaporin-4 as a molecular partner of cystic fibrosis transmembrane conductance regulator in rat Sertoli cells. Biochem. Biophys. Res. Commun. 2014, 446, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Troedsson, M.; Rutllant, J. Region-specific expression of aquaporin subtypes in equine testis, epididymis, and ductus deferens. Anat. Rec. 2013, 296, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Iacovetta, C.; Rudloff, E.; Kirby, R. The role of aquaporin 4 in the brain. Vet. Clin. Pathol. 2012, 41, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.G.; Oliveira, P.F.; Socorro, S.; Moreira, P.I. Impact of diabetes in blood-testis and blood-brain barriers: Resemblances and differences. Curr. Diabetes Rev. 2012, 8, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Tani, T.; Koyama, Y.; Nihei, K.; Hatakeyama, S.; Ohshiro, K.; Yoshida, Y.; Yaoita, E.; Sakai, Y.; Hatakeyama, K.; Yamamoto, T. Immunolocalization of aquaporin-8 in rat digestive organs and testis. Arch. Histol. Cytol. 2001, 64, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Koyama, N.; Ishibashi, K.; Kuwahara, M.; Inase, N.; Ichioka, M.; Sasaki, S.; Marumo, F. Cloning and functional expression of human aquaporin8 cDNA and analysis of its gene. Genomics 1998, 54, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Gravis, C.; Yates, R.; Chen, I. Light and electron microscopic localization of ATPase in normal and degenerating testes of syrian hamsters. Am. J. Anat. 1976, 147, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Song, Y.; Zhao, D.; Verkman, A.S. Phenotype analysis of aquaporin-8 null mice. Am. J. Physiol. Cell Physiol. 2005, 288, C1161–C1170. [Google Scholar] [CrossRef] [PubMed]
- Jesus, T.T.; Bernardino, R.L.; Martins, A.D.; Sá, R.; Sousa, M.; Alves, M.G.; Oliveira, P.F. Aquaporin-9 is expressed in rat Sertoli cells and interacts with the cystic fibrosis transmembrane conductance regulator. IUBMB Life 2014, 66, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Nihei, K.; Koyama, Y.; Tani, T.; Yaoita, E.; Ohshiro, K.; Adhikary, L.P.; Kurosaki, I.; Shirai, Y.; Hatakeyama, K.; Yamamoto, T. Immunolocalization of aquaporin-9 in rat hepatocytes and leydig cells. Arch. Histol. Cytol. 2001, 64, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Svelto, M.; Calamita, G. Possible functional implications of aquaporin water channels in reproductive physiology and medically assisted procreation. Cell. Mol. Biol. 2003, 49, 515–519. [Google Scholar] [PubMed]
- Badaut, J.; Regli, L. Distribution and possible roles of aquaporin 9 in the brain. Neuroscience 2004, 129, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, J.P.; Kowalik, A.; Gallardi, R.L.; Egeler, O.; Clubb, B.H. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in Sertoli cells. J. Androl. 2000, 21, 625–635. [Google Scholar] [PubMed]





| Aquaporin | Subcellular Location | Suggested Functional Involvement | Suggested Clinical Relevance |
|---|---|---|---|
| AQP8 | AM; SAV; SER; IMM | Secretion of canalicular bile water; preservation of cytoplasm osmolarity during glycogen synthesis and degradation; mitochondrial ammonia detoxification and ureagenesis; mitochondrial H2O2 release | Cholestasis |
| AQP9 | BLM | Uptake of glycerol during starvation; import of water from sinusoidal blood; urea extrusion | Cholestasis; T2D; NAFLD; Hepatocellular carcinoma |
| AQP11 | RER | RER homeostasis; liver regeneration | Foam-like hepatocyte |
| Aquaporin | Testicular Distribution | Suggested Function |
|---|---|---|
| AQP0 | Sertoli cells and Leydig cells | Establishment of an adequate luminal environment in the seminiferous tubule; Transport of water from interstitial space into the lumen of the seminiferous tubule, in order to promote the movement of spermatozoa into the epididymal ducts |
| AQP4 | Sertoli cells | Regulation of extracellular space volume, potassium buffering, fluid circulation and reabsorption |
| AQP8 | Sertoli cells and germ cells | Formation of the seminiferous tubular fluid |
| AQP9 | Sertoli cells, Leydig cells, spermatocytes, efferent ducts, epididymis | Transport of water and non-charged solutes in Leydig cells; Formation of the seminiferous luminal fluid |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardino, R.L.; Marinelli, R.A.; Maggio, A.; Gena, P.; Cataldo, I.; Alves, M.G.; Svelto, M.; Oliveira, P.F.; Calamita, G. Hepatocyte and Sertoli Cell Aquaporins, Recent Advances and Research Trends. Int. J. Mol. Sci. 2016, 17, 1096. https://doi.org/10.3390/ijms17071096
Bernardino RL, Marinelli RA, Maggio A, Gena P, Cataldo I, Alves MG, Svelto M, Oliveira PF, Calamita G. Hepatocyte and Sertoli Cell Aquaporins, Recent Advances and Research Trends. International Journal of Molecular Sciences. 2016; 17(7):1096. https://doi.org/10.3390/ijms17071096
Chicago/Turabian StyleBernardino, Raquel L., Raul A. Marinelli, Anna Maggio, Patrizia Gena, Ilaria Cataldo, Marco G. Alves, Maria Svelto, Pedro F. Oliveira, and Giuseppe Calamita. 2016. "Hepatocyte and Sertoli Cell Aquaporins, Recent Advances and Research Trends" International Journal of Molecular Sciences 17, no. 7: 1096. https://doi.org/10.3390/ijms17071096
APA StyleBernardino, R. L., Marinelli, R. A., Maggio, A., Gena, P., Cataldo, I., Alves, M. G., Svelto, M., Oliveira, P. F., & Calamita, G. (2016). Hepatocyte and Sertoli Cell Aquaporins, Recent Advances and Research Trends. International Journal of Molecular Sciences, 17(7), 1096. https://doi.org/10.3390/ijms17071096