Expression and Distribution Pattern of Aquaporin 4, 5 and 11 in Retinas of 15 Different Species
Abstract
:1. Introduction
2. Results
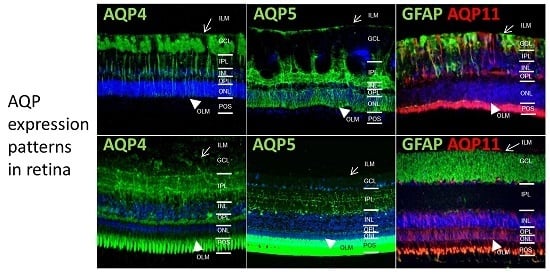
2.1. In Different Species, Aquaporin 4 (AQP4) is Predominantly Expressed at Retinal Muller Glial Cells
2.2. AQP5 Expression Pattern Varies in Retinas of Different Species
2.3. AQP11 Is Primarily Located at Retinal Muller Glial (RMG) Cells and Photoreceptor Outer Segments in Most Retinas Examined
3. Discussion
4. Materials and Methods
4.1. Animal Eye Specimen
4.2. Preparation for Immunohistochemistry
4.3. Immunohistochemical Detection of Aquaporins
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AQP | Aquaporin |
| CNS | Central nervous system |
| DAPI | 4′,6 Diamidino-2-phenylindole |
| GFAP | Glial fibrillary acidic protein |
| IgG | Immunoglobulin G |
| MIP | Major intrinsic protein |
References
- Ishibashi, K.; Kondo, S.; Hara, S.; Morishita, Y. The evolutionary aspects of aquaporin family. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R566–R576. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Fischbarg, J. Water channels and their roles in some ocular tissues. Mol. Asp. Med. 2012, 33, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Ruiz-Ederra, J.; Levin, M.H. Functions of aquaporins in the eye. Prog. Retin. Eye Res. 2008, 27, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Schey, K.L.; Wang, Z.; Wenke, J.L.; Qi, Y. Aquaporins in the eye: Expression, function, and roles in ocular disease. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C. Aquaporin-4 in brain and spinal cord oedema. Neuroscience 2010, 168, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Manley, G.T.; Krishna, S.; Verkman, A.S. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004, 18. [Google Scholar] [CrossRef] [PubMed]
- Hamann, S.; Zeuthen, T.; Cour, M.L.; Nagelhus, E.A.; Ottersen, O.P.; Agre, P.; Nielsen, S. Aquaporins in complex tissues: Distribution of aquaporins 1–5 in human and rat eye. Am. J. Physiol. Cell Physiol. 1998, 274, C1332–C1345. [Google Scholar]
- Da, T.; Verkman, A.S. Aquaporin-4 gene disruption in mice protects against impaired retinal function and cell death after ischemia. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4477–4483. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Sun, J.H.; Xiang, F.F.; Liu, L.; Li, W.J. Aquaporin 4 knockdown exacerbates streptozotocin-induced diabetic retinopathy through aggravating inflammatory response. Exp. Eye Res. 2012, 98, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Wendu, R.L.; Yao, J.; Ren, Y.; Zhao, Y.X.; Cao, G.F.; Qin, J.; Yan, B. Abnormal glutamate metabolism in the retina of aquaporin 4 (AQP4) knockout mice upon light damage. Neurol. Sci. 2014, 35, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, C.; Amann, B.; Feuchtinger, A.; Hauck, S.M.; Deeg, C.A. Differential expression of inwardly rectifying K+ channels and aquaporins 4 and 5 in autoimmune uveitis indicates misbalance in muller glial cell-dependent ion and water homeostasis. Glia 2011, 59, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Deeg, C.A.; Amann, B.; Lutz, K.; Sieglinde, H.; Karina, L.; Elisabeth, K.; Hauck, S.M. Aquaporin 11, a regulator of water efflux at retinal müller glial cell surface decreases concomitant with immune mediated gliosis. J. Neuroinflamm. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Muta, K.; Sonoda, H.; Kato, A.; Abdeen, A.; Ikeda, M. The role of cysteine 227 in subcellular localization, water permeability, and multimerization of aquaporin-11. FEBS Open Bio. 2014, 4, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Role of aquaporin water channels in eye function. Exp. Eye Res. 2003, 76, 137–143. [Google Scholar] [CrossRef]
- Goodyear, M.J.; Crewther, S.G.; Junghans, B.M. A role for aquaporin-4 in fluid regulation in the inner retina. Vis. Neurosci. 2009, 26, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Cusato, K.; Nicchia, G.P.; Frigeri, A.; Spray, D.C. A developmental switch in the expression of aquaporin-4 and KIR4.1 from horizontal to müller cells in mouse retina. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, Y.; Oku, H.; Morishita, S.; Horie, T.; Kida, T.; Mimura, M.; Fukumoto, M.; Kojima, S.; Ikeda, T. Negative impact of AQP-4 channel inhibition on survival of retinal ganglion cells and glutamate metabolism after crushing optic nerve. Exp. Eye Res. 2016, 146, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Amiry-Moghaddam, M.; Ottersen, O.P. The molecular basis of water transport in the brain. Nat. Rev. Neurosci. 2003, 4, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6. [Google Scholar] [CrossRef] [PubMed]
- Hauck, S.M.; Dietter, J.; Kramer, R.L.; Hofmaier, F.; Zipplies, J.K.; Amann, B.; Feuchtinger, A.; Deeg, C.A.; Ueffing, M. Deciphering membrane-associated molecular processes in target tissue of autoimmune uveitis by label-free quantitative mass spectrometry. Mol. Cell. Proteom. 2010, 9, 2292–2305. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.V.; Saito, I.; Yang, X.U.N.; Wax, M.B. Expression of aquaporins in the rat ocular tissue. Exp. Eye Res. 1997, 64, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; Brown, D.; Nielsen, S. Aquaporin water channels: Unanswered questions and unresolved controversies. Curr. Opin. Cell Biol. 1995, 7, 472–483. [Google Scholar] [CrossRef]
- Delporte, C.; Bryla, A.; Perret, J. Aquaporins in salivary glands: From basic research to clinical applications. Int. J. Mol. Sci. 2016, 17, 166. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.; Fuchtbauer, E.M.; Fuchtbauer, A.; Jelen, S.; Malmendal, A.; Fenton, R.A.; Nielsen, S. Liver-specific aquaporin 11 knockout mice show rapid vacuolization of the rough endoplasmic reticulum in periportal hepatocytes after amino acid feeding. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G501–G515. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, D.; Praetorius, J.; Tsunenari, T.; Nielsen, S.; Agre, P. Aquaporin-11: A channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Andoo, A.; Shimono, M.; Takamatsu, N.; Taki, A.; Muta, K.; Matsushita, W.; Uechi, T.; Matsuzaki, T.; Kenmochi, N.; et al. The NPC motif of aquaporin-11, unlike the NPA motif of known aquaporins, is essential for full expression of molecular function. J. Biol. Chem. 2011, 286, 3342–3350. [Google Scholar] [CrossRef] [PubMed]
- Knut, S.; Christine, C.; Fabienne, R. Preclinical studies on specific gene therapy for recessive retinal degenerative diseases. Curr. Gene Ther. 2010, 10, 389–403. [Google Scholar]
- Grosche, A.; Hauser, A.; Lepper, M.F.; Mayo, R.; von Toerne, C.; Merl-Pham, J.; Hauck, S.M. The proteome of native adult muller glial cells from murine retina. Mol. Cell. Proteom. 2016, 15, 462–480. [Google Scholar] [CrossRef] [PubMed]
- Amann, B.; Hirmer, S.; Hauck, S.M.; Kremmer, E.; Ueffing, M.; Deeg, C.A. True blue: S-opsin is widely expressed in different animal species. J. Anim. Physiol. Anim. Nutr. 2014, 98, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Ehrenhofer, M.C.; Deeg, C.A.; Reese, S.; Liebich, H.G.; Stangassinger, M.; Kaspers, B. Normal structure and age-related changes of the equine retina. Vet. Ophthalmol. 2002, 5, 39–47. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amann, B.; Kleinwort, K.J.H.; Hirmer, S.; Sekundo, W.; Kremmer, E.; Hauck, S.M.; Deeg, C.A. Expression and Distribution Pattern of Aquaporin 4, 5 and 11 in Retinas of 15 Different Species. Int. J. Mol. Sci. 2016, 17, 1145. https://doi.org/10.3390/ijms17071145
Amann B, Kleinwort KJH, Hirmer S, Sekundo W, Kremmer E, Hauck SM, Deeg CA. Expression and Distribution Pattern of Aquaporin 4, 5 and 11 in Retinas of 15 Different Species. International Journal of Molecular Sciences. 2016; 17(7):1145. https://doi.org/10.3390/ijms17071145
Chicago/Turabian StyleAmann, Barbara, Kristina J. H. Kleinwort, Sieglinde Hirmer, Walter Sekundo, Elisabeth Kremmer, Stefanie M. Hauck, and Cornelia A. Deeg. 2016. "Expression and Distribution Pattern of Aquaporin 4, 5 and 11 in Retinas of 15 Different Species" International Journal of Molecular Sciences 17, no. 7: 1145. https://doi.org/10.3390/ijms17071145
APA StyleAmann, B., Kleinwort, K. J. H., Hirmer, S., Sekundo, W., Kremmer, E., Hauck, S. M., & Deeg, C. A. (2016). Expression and Distribution Pattern of Aquaporin 4, 5 and 11 in Retinas of 15 Different Species. International Journal of Molecular Sciences, 17(7), 1145. https://doi.org/10.3390/ijms17071145