Aluminum Toxicity-Induced Alterations of Leaf Proteome in Two Citrus Species Differing in Aluminum Tolerance
Abstract
:1. Introduction
2. Results
2.1. Leaf Gas Exchange, Al and Total Soluble Protein Concentrations
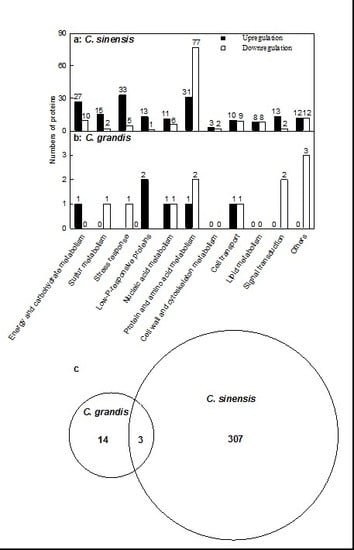
2.2. Leaf Al Toxicity-Responsive Proteins
2.3. Transcriptional Analysis of Genes for Some Differentially-Abundant Proteins
2.4. Analysis of Five Al Toxicity-Responsive Enzymes in C. sinensis Leaves
3. Discussion
3.1. C. sinensis Displayed Higher Metabolic Flexibility than C. grandis
3.2. Al Toxicity-Induced Alterations of Energy and Carbohydrate Metabolism-Related Proteins Contribute to the Higher Al-Tolerance of C. sinensis
3.3. Al Toxicity-Induced Upregulation of Antioxidant Systems and Other Stress-Related Proteins Played a Role in the Al Tolerance of C. sinensis
3.4. Low P-Responsive Proteins Were Induced by Al Toxicity, Particularly in C. sinensis Leaves
3.5. RNA Regulations Might Play a Role in the Higher Al Tolerance of C. sinensis
3.6. Protein Metabolism Was More Adaptive to Al Toxicity in C. sinensis than in C. grandis
3.7. Cell Wall and Cytoskeleton Metabolism-Related Proteins
3.8. Cellular Transport-Related Proteins
3.9. Lipid Metabolism-Related Proteins
3.10. Signal Transduction-Related Proteins
4. Materials and Methods
4.1. Plant Materials and Al Treatments
4.2. Measurements of Leaf Gas Exchange, Total Soluble Protein and Al Concentrations
4.3. Protein Extraction
4.4. iTRAQ Analysis
4.5. qRT-PCR Analysis of Gene Expression
4.6. Analysis of SOD, APX, CAT, MDAR and LOX Activities in C. sinensis Leaves
4.7. Experimental Design and Statistical Analysis
4.8. Data Deposit
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Foy, C.D.; Chaney, R.L.; White, W.C. The physiology of metal toxicity in plants. Annu. Rev. Plant Physiol. 1978, 29, 511–566. [Google Scholar] [CrossRef]
- Kinraide, T.B. Identity of the rhizotoxic aluminium species. Plant Soil 1991, 134, 167–178. [Google Scholar]
- Kochian, L.V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- Yang, L.T.; Qi, Y.P.; Jiang, H.X.; Chen, L.S. Roles of organic acid anion secretion in aluminium tolerance of higher plants. BioMed Res. Int. 2013, 2013, 173682. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Fu, Y.Q.; Yu, Z.W.; Shen, H. Status of red soil acidification and aluminum toxicity in south China and prevention. Soils 2013, 45, 577–584. [Google Scholar]
- Wang, L.Q.; Yang, L.T.; Guo, P.; Zhou, X.X.; Ye, X.; Chen, E.J.; Chen, L.S. Leaf cDNA-AFLP analysis reveals novel mechanisms for boron-induced alleviation of aluminum toxicity in Citrus grandis seedlings. Ecotox. Environ. Saf. 2015, 120, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Lan, P.; Shen, R.F.; Li, W.F. Proteomics of aluminum tolerance in plants. Proteomics 2014, 14, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.X.; Yang, L.T.; Qi, Y.P.; Guo, P.; Chen, L.S. Mechanisms on boron-induced alleviation of aluminum toxicity in Citrus grandis seedlings at a transcriptional level revealed by cDNA-AFLP analysis. PLoS ONE 2015, 10, e0115485. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, H.; Zhu, Y.; Zou, J.; Zhao, F.J.; Huang, C.F. Genome-wide transcriptomic and phylogenetic analyses reveal distinct aluminum tolerance mechanisms in the aluminum-accumulating species buckwheat (Fagopyrum tataricum). BMC Plant Biol. 2015, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.X.; Yang, L.T.; Qi, Y.P.; Lu, Y.B.; Huang, Z.R.; Chen, L.S. Root iTRAQ protein profile analysis of two citrus species differing in aluminum tolerance in response to long-term aluminum toxicity. BMC Genom. 2015, 16, 949. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Xu, X.Y.; Gong, Q.Q.; Xie, C.; Fan, W.; Yang, J.L.; Lin, Q.S.; Zheng, S.J. Root proteome of rice studied by iTRAQ provides integrated insight into aluminum stress tolerance mechanisms in plants. J. Proteom. 2014, 98, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Y.; Zhang, J.; Shi, W.; Qian, C.; Peng, X. Identification of aluminum-responsive proteins in rice roots by a proteomic approach: Cysteine synthase as a key player in Al response. Proteomics 2013, 7, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tian, D.; Todd, C.D.; Luo, Y.; Hu, X. Comparative proteome analyses reveal that nitric oxide is an important signal molecule in the response of rice to aluminum toxicity. J. Proteome Res. 2013, 12, 1316–1330. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Qi, J.L.; Wang, S.S.; Su, J.; Xu, G.H.; Zhang, M.S.; Miao, L.; Peng, X.X.; Tian, D.; Yang, Y.H. Comparative proteome analysis of differentially expressed proteins induced by Al toxicity in soybean. Physiol. Plant. 2007, 131, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Duressa, D.; Soliman, K.; Taylor, R.; Senwo, Z. Proteomic analysis of soybean roots under aluminum stress. J. Plant Genom. 2011, 2011, 282531. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Cao, F.; Chen, X.; Zhang, M.; Ahmed, I.M.; Chen, Z.H.; Li, C.; Zhang, G.; Wu, F. Comparative proteomic analysis of aluminum tolerance in Tibetan wild and cultivated barleys. PLoS ONE 2013, 8, e63428. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sauvé, R.; Thannhauser, T.W. Proteome changes induced by aluminium stress in tomato roots. J. Exp. Bot. 2009, 60, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.W.; Roy, S.K.; Kamal, A.H.; Cho, K.; Cho, S.W.; Park, C.S.; Choi, J.S.; Komatsu, S.; Woo, S.H. Proteome analysis of roots of wheat seedlings under aluminum stress. Mol. Biol. Rep. 2014, 41, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Kim, Y.G.; Lee, B.H. Proteomic response of alfalfa subjected to aluminum (Al) stress at low pH soil. J. Korean Grassl. Forage Sci. 2014, 34, 262–268. [Google Scholar] [CrossRef]
- Lin, Z.; Myhre, D.L. Citrus root growth as affected by soil aluminum level under field conditions. Soil Sci. Soc. Am. J. 1990, 54, 1340–1344. [Google Scholar] [CrossRef]
- Li, Y.; Han, M.Q.; Lin, F.; Ten, Y.; Lin, J.; Zhu, D.H.; Guo, P.; Weng, Y.B.; Chen, L.S. Soil chemical properties, ‘Guanximiyou’ pummelo leaf mineral nutrient status and fruit quality in the southern region of Fujian province, China. J. Soil Sci. Plant Nutr. 2015, 15, 615–628. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Li, J.; Wu, S.H.; Pang, D.M. Nutrition condition of the orchards in the main production areas of Guanxi honeypomelo trees (Pinhe county). J. Fujian Agric. Univ. 2001, 30, 40–43. [Google Scholar]
- Yang, L.T.; Jiang, H.X.; Tang, N.; Chen, L.S. Mechanisms of aluminum tolerance in two species of citrus: Secretion of organic acid anions and immobilization of aluminum by phosphorus in roots. Plant Sci. 2011, 180, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Baena-González, E.; Sheen, J. Convergent energy and stress signaling. Trends Plant Sci. 2008, 13, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Qi, Y.P.; Smith, B.R.; Liu, X.H. Aluminum-induced decrease in CO2 assimilation in citrus seedlings is unaccompanied by decreased activities of key enzymes involved in CO2 assimilation. Tree Physiol. 2005, 25, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Minic, Z.; Jouanin, L. Plant glycosyl hydrolases involved in cell wall polysaccharide degradation. Plant Physiol. Biochem. 2006, 44, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Minic, Z. Physiological roles of plant glycoside hydrolases. Planta 2008, 227, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Xiao, X.; Dong, Z.; Chen, Y. Silicon effects on antioxidative enzymes and lipid peroxidation in leaves and roots of peanut under aluminum stress. Acta Physiol. Plant. 2014, 36, 3063–3069. [Google Scholar] [CrossRef]
- Yin, L.; Mano, J.; Wang, S.; Tsuji, W.; Tanaka, K. The involvement of lipid peroxide-derived aldehydes in aluminum toxicity of tobacco roots. Plant Physiol. 2010, 152, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Gill, R.; Kaushik, M.; Hasanuzzaman, M.; Pereira, E.; Ahmad, I.; Tuteja, N.; Gill, S.S. ATP-sulfurylase, sulfur-compounds, and plant stress tolerance. Front. Plant Sci. 2015, 6, 210. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, B.; Gardner, R.C.; Ezaki, Y.; Matsumoto, H. Expression of aluminum induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol. 2000, 122, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Qi, Y.P.; Liu, X.H. Effects of aluminum on light energy utilization and photoprotective systems in citrus leaves. Ann. Bot. 2005, 96, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, R.; Bartels, D.; Kirch, H.H. Overexpression of a stress-inducible aldehyde dehydrogenase gene from Arabidopsis thaliana in transgenic plants improves stress tolerance. Plant J. 2003, 35, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Turóczy, Z.; Kis, P.; Török, K.; Cserháti, M.; Lendvai, A.; Dudits, D.; Horváth, G.V. Overproduction of a rice aldo-keto reductase increases oxidative and heat stress tolerance by malondialdehyde and methylglyoxal detoxification. Plant Mol. Biol. 2011, 75, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Liu, S.; Luo, R.; Gong, P.; Zhao, D.; Fang, X. Cloning and expression of an alcohol dehydrogenase from Lotus japonicus and characterization of LjADH1. Legume Genom. Genet. 2011, 2, 6–13. [Google Scholar]
- Karuppanapandian, T.; Rhee, S.J.; Kim, E.J.; Han, B.K.; Hoekenga, O.A.; Lewe, G.P. Proteomic analysis of differentially expressed proteins in the roots of Columbia-0 and Landsberg erecta ecotypes of Arabidopsis thaliana in response to aluminum toxicity. Can. J. Plant Sci. 2012, 92, 1267–1282. [Google Scholar] [CrossRef]
- Jiang, H.X.; Tang, N.; Zheng, J.G.; Li, Y.; Chen, L.S. Phosphorus alleviates aluminum-induced inhibition of growth and photosynthesis in Citrus grandis seedlings. Physiol. Plant. 2009, 137, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Domíguez, E.E.; Valencia-Turcotte, L.G.; Rodríguez-Sotres, R. Changes in expression of soluble inorganic pyrophosphatases of Phaseolus vulgaris under phosphate starvation. Plant Sci. 2012, 187, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Bariola, P.A.; Howard, C.J.; Taylor, C.B.; Verburg, M.T.; Jaglan, V.D.; Green, P.J. The Arabidopsis ribonuclease gene RNS1 is tightly controlled in response to phosphate limitation. Plant J. 1994, 6, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhou, W.; El Sheery, N.I.; Peters, C.; Li, M.; Wang, X.; Huang, J. Characterization of the Arabidopsis glycerophosphodiester phosphodiesterase (GDPD) family reveals a role of the plastid-localized AtGDPD1 in maintaining cellular phosphate homeostasis under phosphate starvation. Plant J. 2011, 66, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.L.; Hurley, B.A.; Tran, H.T.; Valentine, A.J.; She, Y.M.; Knowles, V.L.; Plaxton, W.C. In vivo regulatory phosphorylation of the phosphoenolpyruvate carboxylase AtPPC1 in phosphate-starved Arabidopsis thaliana. Biochem. J. 2009, 420, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Caparrós-Martín, J.A.; McCarthy-Suárez, I.; Culiáñez-Macià, F.A. HAD hydrolase function unveiled by substrate screening: Enzymatic characterization of Arabidopsis thaliana subclass I phosphosugar phosphatase AtSgpp. Planta 2013, 237, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Delhaize, E.; Ma, J.F.; Ryan, P.R. Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci. 2012, 17, 341–348. [Google Scholar] [CrossRef] [PubMed]
- García-Oliveira, A.L.; Benito, C.; Prieto, P.; de Andrade Menezes, R.; Rodrigues-Pousada, C.; Guedes-Pinto, H.; Martins-Lopes, P. Molecular characterization of TaSTOP1 homoeologues and their response to aluminium and proton (H+) toxicity in bread wheat (Triticum aestivum L.). BMC Plant Biol. 2013, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, S.; Koyama, H.; Iuchi, A.; Kobayashi, A.; Kitabayashi, S.; Kobayashi, Y.; Ikka, T.; Hirayama, T.; Shinozaki, K.; Kobayashi, M. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 9900–9905. [Google Scholar] [CrossRef] [PubMed]
- Duressa, D.; Soliman, K.M.; Taylor, R.W.; Chen, D. Gene expression profiling in soybean under aluminum stress: Genes differentially expressed between Al-tolerant and Al-sensitive genotypes. Am. J. Mol. Biol. 2011, 1, 156–173. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, Y.; Zhuo, C.; Lu, S.; Guo, Z. Overexpression of a NF-YC transcription factor from bermudagrass confers tolerance to drought and salinity in transgenic rice. Plant Biotechnol. J. 2015, 13, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.E.; Repetti, P.P.; Adams, T.R.; Creelman, R.A.; Wu, J.; Warner, D.C.; Anstrom, D.C.; Bensen, R.J.; Castiglioni, P.P.; Donnarummo, M.G.; et al. Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc. Natl. Acad. Sci. USA 2007, 104, 16450–16455. [Google Scholar] [CrossRef] [PubMed]
- Floris, M.; Mahgoub, H.; Lanet, E.; Robaglia, C.; Menand, B. Post-transcriptional regulation of gene expression in plants during abiotic stress. Int. J. Mol. Sci. 2009, 10, 3168–3185. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Piñeros, M.A.; Kochian, L.V. The role of aluminum sensing and signaling in plant aluminum resistance. J. Integr. Plant Biol. 2014, 56, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.C.; Mei, C.; Liang, S.; Yu, Y.T.; Lu, K.; Wu, Z.; Wang, X.F.; Zhang, D.P. Crucial roles of the pentatricopeptide repeat protein SOAR1 in Arabidopsis response to drought, salt and cold stresses. Plant Mol. Biol. 2015, 88, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.X.; Huang, C.; Guo, G.Q.; Yang, Z.N. Roles of the nuclear encoded chloroplast SMR domain-containing PPR protein SVR7 in photosynthesis and oxidative stress tolerance in Arabidopsis. J. Plant Biol. 2014, 57, 291–301. [Google Scholar] [CrossRef]
- Lee, B.H.; Kapoor, A.; Zhu, J.; Zhu, J.K. STABILIZED1, a stress-upregulated nuclear protein, is required for pre-mRNA splicing, mRNA turnover, and stress tolerance in Arabidopsis. Plant Cell 2006, 18, 1736–1749. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.F.; Gao, J.X.; Shu, J.M. Study on the response of Pinus massoniana seedling to aluminum. Acta Ecol. Sin. 1992, 12, 239–246. [Google Scholar]
- Hait, W.N.; Versele, M.; Yang, J.M. Surviving metabolic stress: Of mice (squirrels) and men. Cancer Discov. 2014, 4, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Taylor, G.J.; Deyholos, M.K. Transcriptomic responses to aluminum stress in roots of Arabidopsis thaliana. Mol. Genet. Genom. 2008, 279, 339–357. [Google Scholar] [CrossRef] [PubMed]
- García-Lorenzo, M.; Sjödin, A.; Jansson, S.; Funk, C. Protease gene families in Populus and Arabidopsis. BMC Plant Biol. 2006, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Lyzenga, W.J.; Stone, S.L. Abiotic stress tolerance mediated by protein ubiquitination. J. Exp. Bot. 2012, 63, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Link, B.M.; Cosgrove, D.J. Acid-growth response and α-expansins in suspension cultures of bright yellow 2 tobacco. Plant Physiol. 1988, 118, 907–916. [Google Scholar] [CrossRef]
- Zhang, J.; He, Z.; Tian, H.; Zhu, G.; Peng, X. Identification of aluminium-responsive genes in rice cultivars with different aluminium sensitivities. J. Exp. Bot. 2007, 58, 2269–2278. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Christensen, H.E.M.; Ishimaru, Y.; Dong, C.H.; Wen, C.M.; Cleary, A.L.; Chua, N.H. Profilin plays a role in cell elongation, cell shape maintenance, and flowering in Arabidopsis. Plant Physiol. 2000, 124, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.A.; Good, A.G.; Taylor, J.T. Induction of vacuolar ATPase and mitochondrial ATP synthase by aluminum in an aluminum-resistant cultivar of wheat. Plant Physiol. 2001, 125, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.A.; Good, A.G.; Taylor, J.T. Vacuolar H+-ATPase, but not mitochondrial F1F0-ATPase, is required for aluminum resistance in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2001, 205, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.F.; Ravet, K.; Arnaud, N.; Duc, C.; Boucherez, J.; Touraine, B. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann. Bot. 2010, 105, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Lira-Ruan, V.; Ross, E.J.H.; Sarath, G.; Klucas, R.V.; Arredondo-Peter, R. Mapping and analysis of a hemoglobin gene family from Oryza sativa. Plant Physiol. Biochem. 2002, 40, 199–202. [Google Scholar] [CrossRef]
- Vigeolas, H.; Hühn, D.; Geigenberger, P. Nonsymbiotic hemoglobin-2 leads to an elevated energy state and to a combined increase in polyunsaturated fatty acids and total oil content when overexpressed in developing seeds of transgenic Arabidopsis plants. Plant Physiol. 2011, 155, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Hill, R.D. Mitochondrial respiration and hemoglobin gene expression in barley aleurone tissue. Plant Physiol. 1997, 114, 835–840. [Google Scholar] [PubMed]
- Hunt, P.W.; Klok, E.J.; Trevaskis, B.; Watts, R.A.; Ellis, M.H.; Peacock, W.J.; Dennis, E.S. Increased level of hemoglobin 1 enhances survival of hypoxic stress and promotes early growth in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2002, 99, 17197–17202. [Google Scholar] [CrossRef] [PubMed]
- Nechushtai, R.; Conlan, A.R.; Harir, Y.; Song, L.; Yogev, O.; Eisenberg-Domovich, Y.; Livnah, O.; Michaeli, D.; Rosen, R.; Ma, V.; et al. Characterization of Arabidopsis NEET reveals an ancient role for NEET proteins in iron metabolism. Plant Cell 2007, 24, 2139–2154. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yan, X.; Chen, Q.; Jiang, N.; Fu, W.; Ma, B.; Liu, J.; Li, C.; Bednarek, S.Y.; Pan, J. Clathrin light chains regulate clathrin-mediated trafficking, auxin signaling, and development in Arabidopsis. Plant Cell 2013, 25, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K.; Cai, Y.; Hillmer, S.; Robinson, D.G.; Jiang, L.W. SCAMPs highlight the developing cell plate during cytokinesis in tobacco BY-2 cells. Plant Physiol. 2008, 147, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Chen, Z.; Yang, X.; Liu, G. Arabidopsis voltage-dependent anion channel 1 (AtVDAC1) is required for female development and maintenance of mitochondrial functions related to energy-transaction. PLoS ONE 2014, 9, e106941. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.H.; Yu, G.; Li, J.T.; Jia, P.; Zhang, J.C.; Jia, C.G.; Zhang, Y.H.; Pan, H.Y. A heavy metal-associated protein (AcHMA1) from the halophyte, Atriplex canescens (Pursh) Nutt.; confers tolerance to iron and other abiotic stresses when expressed in Saccharomyces cerevisiae. Int. J. Mol. Sci. 2014, 15, 14891–14906. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Peel, G.J.; Lei, Z.; Aziz, N.; Dai, X.; He, J.; Watson, B.; Zhao, P.X.; Sumner, L.W.; Dixon, R.A. Transcript and proteomic analysis of developing white lupin (Lupinus albus L.) roots. BMC Plant Biol. 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Santino, A.; Taurino, M.; De Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, P.H.P.; Cambraia, J.; Sant’Anna, R.; Mosquim, P.R.; Moreira, M.A. Aluminum effects on lipid peroxidation and on the activities of enzymes of oxidative metabolism in sorghum. R. Bras. Fisiol. Veg. 1999, 11, 137–143. [Google Scholar]
- Li, C.; Schilmiller, A.L.; Liu, G.; Lee, G.I.; Jayanty, S.; Sageman, C.; Vrebalov, J.; Giovannoni, J.J.; Yagi, K.; Kobayashi, Y.; et al. Role of β-oxidation in jasmonate biosynthesis and systemic wound signaling in tomato. Plant Cell 2005, 17, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, M.A.; Forment, J.; Roldan, M.; Serrano, R.; Vicente, O. Overexpression of Arabidopsis thaliana LTL1, a salt-induced gene encoding a GDSL-motif lipase, increases salt tolerance in yeast and transgenic plants. Plant Cell Environ. 2006, 29, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Gujjar, R.S.; Akhtar, M.; Rai, A.; Singh, M. Expression analysis of drought induced genes in wild tomato line (Solanum habrochaites). Curr. Sci. 2014, 107, 496–502. [Google Scholar]
- Clay, N.K.; Nelson, T. VH1, a provascular cell-specific receptor kinase that influences leaf cell patterns in Arabidopsis. Plant Cell 2002, 14, 2707–2722. [Google Scholar] [CrossRef] [PubMed]
- País, S.M.; Téllez-Iñón, M.; Capiati, D.A. Serine/threonine protein phosphatases type 2A and their roles in stress signaling. Plant Signal. Behav. 2009, 4, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, S.Y.; Kim, W.Y.; Jung, Y.J.; Chae, H.B.; Jung, H.S.; Kang, C.H.; Shin, M.R.; Kim, S.Y.; Suudi, M.; et al. Heat-induced chaperone activity of serine/threonine protein phosphatase 5 enhances thermotolerance in Arabidopsis thaliana. New Phytol. 2011, 191, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hsu, P.H. Effect of initial pH, phosphate, and silicate on the determination of aluminum with aluminon. Soil Sci. 1963, 96, 230–238. [Google Scholar] [CrossRef]
- Yang, L.T.; Qi, Y.P.; Lu, Y.B.; Guo, P.; Sang, W.; Feng, H.; Zhang, H.X.; Chen, L.S. iTRAQ protein profile analysis of Citrus sinensis roots in response to long-term boron-deficiency. J. Proteom. 2013, 93, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.T.; Lu, Y.B.; Zhang, Y.; Guo, P.; Chen, L.S. Proteomic profile of Citrus grandis roots under long-term boron-deficiency revealed by iTRAQ. Trees Struct. Funct. 2016, 30, 1057–1071. [Google Scholar] [CrossRef]
- Gan, C.S.; Chong, P.K.; Pham, T.K.; Wright, P.C. Technical, experimental, and biological variations in isobaric tags for relative and absolute quantitation (iTRAQ). J. Proteome Res. 2007, 6, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.P.; Qi, Y.P.; You, X.; Yang, L.T.; Guo, P.; Ye, X.; Zhou, X.X.; Ke, F.J.; Chen, L.S. Leaf cDNA-AFLP analysis of two citrus species differing in manganese tolerance in response to long-term manganese toxicity. BMC Genom. 2013, 14, 621. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, L.S.; Jiang, H.X.; Tang, N.; Yang, L.T.; Lin, Z.H.; Li, Y.; Yang, G.H. Effects of manganese-excess on CO2 assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport of leaves, and antioxidant systems of leaves and roots in Citrus grandis seedlings. BMC Plant Biol. 2010, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, B.; Cheesbrough, T.M.; Laakso, S. Lipoxygenase from soybeans. Methods Enzymol. 1981, 71, 441–451. [Google Scholar]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Yang, L.-T.; Qi, Y.-P.; Guo, P.; Lu, Y.-B.; Chen, L.-S. Aluminum Toxicity-Induced Alterations of Leaf Proteome in Two Citrus Species Differing in Aluminum Tolerance. Int. J. Mol. Sci. 2016, 17, 1180. https://doi.org/10.3390/ijms17071180
Li H, Yang L-T, Qi Y-P, Guo P, Lu Y-B, Chen L-S. Aluminum Toxicity-Induced Alterations of Leaf Proteome in Two Citrus Species Differing in Aluminum Tolerance. International Journal of Molecular Sciences. 2016; 17(7):1180. https://doi.org/10.3390/ijms17071180
Chicago/Turabian StyleLi, Huan, Lin-Tong Yang, Yi-Ping Qi, Peng Guo, Yi-Bin Lu, and Li-Song Chen. 2016. "Aluminum Toxicity-Induced Alterations of Leaf Proteome in Two Citrus Species Differing in Aluminum Tolerance" International Journal of Molecular Sciences 17, no. 7: 1180. https://doi.org/10.3390/ijms17071180
APA StyleLi, H., Yang, L. -T., Qi, Y. -P., Guo, P., Lu, Y. -B., & Chen, L. -S. (2016). Aluminum Toxicity-Induced Alterations of Leaf Proteome in Two Citrus Species Differing in Aluminum Tolerance. International Journal of Molecular Sciences, 17(7), 1180. https://doi.org/10.3390/ijms17071180