MicroRNA-155 Mediates Augmented CD40 Expression in Bone Marrow Derived Plasmacytoid Dendritic Cells in Symptomatic Lupus-Prone NZB/W F1 Mice
Abstract
:1. Introduction
2. Results
2.1. Lupus Disease Has Limited Impact on Plasmacytoid Dendritic Cells (pDC) Generation Potential of Bone Marrow (BM) Progenitor Cells in NZB/W F1 Mice
2.2. BM-Derived pDCs Display Similar Phenotypes Irrespective of Lupus Disease Stage
2.3. BM-Derived pDCs from Symptomatic Lupus Mice Show Heightened TLR7-Mediated Antigen Presentation and Costimulatory Molecules Expressions
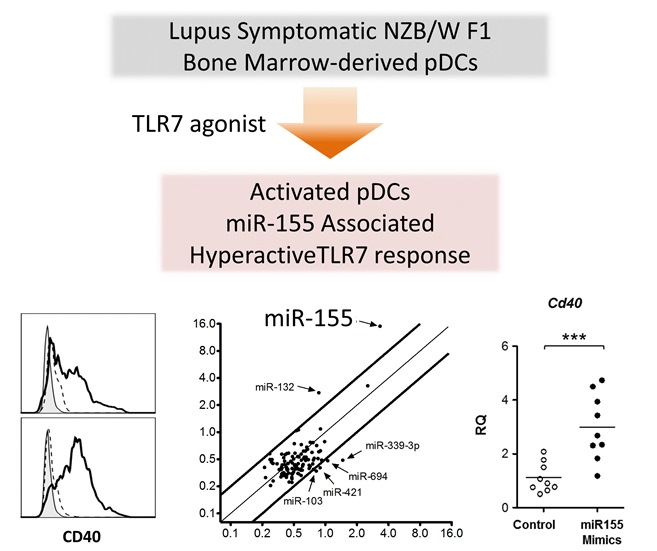
2.4. TLR7 Mediated Overexpression of miR-155 in Lupus pDCs Contributes to the Heightened Cd40 Expression
3. Discussion
4. Experimental Section
4.1. Mice
4.2. pDC Culture, Isolation and Stimulation
4.3. Transfection of miRNA Mimics
4.4. Quantitative Real-Time RT-PCR
4.5. MicroRNA Profiling
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, Y.J. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005, 23, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Gilliet, M.; Cao, W.; Liu, Y.J. Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008, 8, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, A.A.; Sturfelt, G.; Truedsson, L.; Blomberg, J.; Alm, G.; Vallin, H.; Ronnblom, L. Activation of type i interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus 2000, 9, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Baechler, E.C.; Batliwalla, F.M.; Karypis, G.; Gaffney, P.M.; Ortmann, W.A.; Espe, K.J.; Shark, K.B.; Grande, W.J.; Hughes, K.M.; Kapur, V.; et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA 2003, 100, 2610–2615. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.; Palucka, A.K.; Arce, E.; Cantrell, V.; Borvak, J.; Banchereau, J.; Pascual, V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 2003, 197, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Rowland, S.L.; Riggs, J.M.; Gilfillan, S.; Bugatti, M.; Vermi, W.; Kolbeck, R.; Unanue, E.R.; Sanjuan, M.A.; Colonna, M. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J. Exp. Med. 2014, 211, 1977–1991. [Google Scholar] [CrossRef] [PubMed]
- Sisirak, V.; Ganguly, D.; Lewis, K.L.; Couillault, C.; Tanaka, L.; Bolland, S.; D’Agati, V.; Elkon, K.B.; Reizis, B. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J. Exp. Med. 2014, 211, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Rao, D.S.; Chaudhuri, A.A.; Baltimore, D. Physiological and pathological roles for micrornas in the immune system. Nat. Rev. Immunol. 2010, 10, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ranade, K.; Talker, R.; Jallal, B.; Shen, N.; Yao, Y. Microrna-mediated regulation of innate immune response in rheumatic diseases. Arthritis Res. Ther. 2013, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Yim, L.Y.; Lu, L.; Lau, C.S.; Chan, V.S. Microrna regulation in systemic lupus erythematosus pathogenesis. Immune Netw. 2014, 14, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Luo, X.; Cui, H.; Ni, X.; Yuan, M.; Guo, Y.; Huang, X.; Zhou, H.; de Vries, N.; Tak, P.P.; et al. Microrna-146a contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009, 60, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Charrier, E.; Cordeiro, P.; Cordeau, M.; Dardari, R.; Michaud, A.; Harnois, M.; Merindol, N.; Herblot, S.; Duval, M. Post-transcriptional down-regulation of toll-like receptor signaling pathway in umbilical cord blood plasmacytoid dendritic cells. Cell Immunol. 2012, 276, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, P.; Lin, L.; Liu, X.; Ma, F.; An, H.; Wang, Z.; Cao, X. MicroRNA-146a feedback inhibits RIG-I-dependent type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J. Immunol. 2009, 183, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Huang, X.; Cui, H.; Luo, X.; Tang, Y.; Chen, S.; Wu, L.; Shen, N. miR-155 and its star-form partner miR-155* cooperatively regulate type i interferon production by human plasmacytoid dendritic cells. Blood 2010, 116, 5885–5894. [Google Scholar] [CrossRef] [PubMed]
- Agudo, J.; Ruzo, A.; Tung, N.; Salmon, H.; Leboeuf, M.; Hashimoto, D.; Becker, C.; Garrett-Sinha, L.A.; Baccarini, A.; Merad, M.; et al. The miR-126–VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat. Immunol. 2014, 15, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Wu, J.; Zhao, J.; Wang, H.; Liu, Y.; Chen, T.; Kan, X.; Tao, Q.; Shen, X.; Yan, K.; et al. miR-29b and miR-29c are involved in toll-like receptor control of glucocorticoid-induced apoptosis in human plasmacytoid dendritic cells. PLoS ONE 2013, 8, e69926. [Google Scholar] [CrossRef] [PubMed]
- Jin, O.; Kavikondala, S.; Sun, L.; Fu, R.; Mok, M.Y.; Chan, A.; Yeung, J.; Lau, C.S. Systemic lupus erythematosus patients have increased number of circulating plasmacytoid dendritic cells, but decreased myeloid dendritic cells with deficient CD83 expression. Lupus 2008, 17, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Jin, O.; Kavikondala, S.; Mok, M.Y.; Sun, L.; Gu, J.; Fu, R.; Chan, A.; Yeung, J.; Nie, Y.; Lau, C.S. Abnormalities in circulating plasmacytoid dendritic cells in patients with systemic lupus erythematosus. Arthritis Res. Ther. 2010, 12, R137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, Y.J.; Mok, M.Y.; Chan, G.C.; Chan, A.W.; Jin, O.U.; Kavikondala, S.; Lie, A.K.; Lau, C.S. Phenotypic and functional abnormalities of bone marrow-derived dendritic cells in systemic lupus erythematosus. Arthritis Res. Ther. 2010, 12, R91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voulgarelis, M.; Giannouli, S.; Tasidou, A.; Anagnostou, D.; Ziakas, P.D.; Tzioufas, A.G. Bone marrow histological findings in systemic lupus erythematosus with hematologic abnormalities: A clinicopathological study. Am. J. Hematol. 2006, 81, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Zhou, K.X.; Feng, X.B.; Zhang, H.Y.; Ding, X.Q.; Jin, O.; Lu, L.W.; Lau, C.S.; Hou, Y.Y.; Fan, L.M. Abnormal surface markers expression on bone marrow cd34+ cells and correlation with disease activity in patients with systemic lupus erythematosus. Clin. Rheumatol. 2007, 26, 2073–2079. [Google Scholar] [CrossRef] [PubMed]
- Wanitpongpun, C.; Teawtrakul, N.; Mahakkanukrauh, A.; Siritunyaporn, S.; Sirijerachai, C.; Chansung, K. Bone marrow abnormalities in systemic lupus erythematosus with peripheral cytopenia. Clin. Exp. Rheumatol. 2012, 30, 825–829. [Google Scholar] [PubMed]
- Brasel, K.; De Smedt, T.; Smith, J.L.; Maliszewski, C.R. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood 2000, 96, 3029–3039. [Google Scholar] [PubMed]
- Nakano, H.; Yanagita, M.; Gunn, M.D. Cd11c+b220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 2001, 194, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Blasius, A.L.; Giurisato, E.; Cella, M.; Schreiber, R.D.; Shaw, A.S.; Colonna, M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 2006, 177, 3260–3265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Raper, A.; Sugita, N.; Hingorani, R.; Salio, M.; Palmowski, M.J.; Cerundolo, V.; Crocker, P.R. Characterization of siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood 2006, 107, 3600–3608. [Google Scholar] [CrossRef] [PubMed]
- Gilliet, M.; Boonstra, A.; Paturel, C.; Antonenko, S.; Xu, X.L.; Trinchieri, G.; O′Garra, A.; Liu, Y.J. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 2002, 195, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Rice, C.M. Interferon-stimulated genes and their antiviral effector functions. Curr. Opin. Virol. 2011, 1, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ma, J.; Xiao, C.; Han, X.; Qiu, R.; Wang, Y.; Zhou, Y.; Wu, L.; Huang, X.; Shen, N. Phenotypic and functional alterations of pDCs in lupus-prone mice. Sci. Rep. 2016, 6, 20373. [Google Scholar] [CrossRef] [PubMed]
- Diebold, S.S.; Massacrier, C.; Akira, S.; Paturel, C.; Morel, Y.; Reis e Sousa, C. Nucleic acid agonists for toll-like receptor 7 are defined by the presence of uridine ribonucleotides. Eur. J. Immunol. 2006, 36, 3256–3267. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, D.; Paturel, C.; Morel, Y.; Uematsu, S.; Akira, S.; Diebold, S.S. Plasmacytoid dendritic cell-derived type i interferon is crucial for the adjuvant activity of toll-like receptor 7 agonists. Blood 2010, 115, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Chaudhuri, A.A.; Rao, D.S.; Baltimore, D. Inositol phosphatase ship1 is a primary target of miR-155. Proc. Natl. Acad. Sci. USA 2009, 106, 7113–7118. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Sun, L.; Hao, S.; Li, X.; Xu, Y.; Hou, Y. Estrogen modulates bone marrow-derived DCs in sle murine model-(NZB × NZW) F1 female mice. Immunol. Investig. 2008, 37, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Bave, U.; Magnusson, M.; Eloranta, M.L.; Perers, A.; Alm, G.V.; Ronnblom, L. FCΓRIIa is expressed on natural IFN-α-producing cells (plasmacytoid dendritic cells) and is required for the IFN-α production induced by apoptotic cells combined with lupus IgG. J. Immunol. 2003, 171, 3296–3302. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra19. [Google Scholar] [CrossRef] [PubMed]
- Guiducci, C.; Tripodo, C.; Gong, M.; Sangaletti, S.; Colombo, M.P.; Coffman, R.L.; Barrat, F.J. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J. Exp. Med. 2010, 207, 2931–2942. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, P.; Deane, J.A.; Difilippantonio, M.J.; Tarasenko, T.; Satterthwaite, A.B.; Bolland, S. Autoreactive b cell responses to rna-related antigens due to Tlr7 gene duplication. Science 2006, 312, 1669–1672. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Tus, K.; Li, Q.Z.; Wang, A.; Tian, X.H.; Zhou, J.; Liang, C.; Bartov, G.; McDaniel, L.D.; Zhou, X.J.; et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl. Acad. Sci. USA 2006, 103, 9970–9975. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Raber, M.L.; Dunand-Sauthier, I.; Wu, T.; Li, Q.Z.; Uematsu, S.; Akira, S.; Reith, W.; Mohan, C.; Kotzin, B.L.; Izui, S. Critical role of Tlr7 in the acceleration of systemic lupus erythematosus in Tlr9-deficient mice. J. Autoimmun. 2010, 34, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Colonna, L.; Dinnall, J.A.; Shivers, D.K.; Frisoni, L.; Caricchio, R.; Gallucci, S. Abnormal costimulatory phenotype and function of dendritic cells before and after the onset of severe murine lupus. Arthritis Res. Ther. 2006, 8, R49. [Google Scholar] [CrossRef] [PubMed]
- Gleisner, M.A.; Reyes, P.; Alfaro, J.; Solanes, P.; Simon, V.; Crisostomo, N.; Sauma, D.; Rosemblatt, M.; Bono, M.R. Dendritic and stromal cells from the spleen of lupic mice present phenotypic and functional abnormalities. Mol. Immunol. 2013, 54, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.; Genin, A.; Brunner, M.; Scalzi, L.V.; Mishra, N.; Beukelman, T.; Cron, R.Q. Prolonged expression of CD154 on CD4 T cells from pediatric lupus patients correlates with increased CD154 transcription, increased nuclear factor of activated T cell activity, and glomerulonephritis. Arthritis Rheum. 2010, 62, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Goules, A.; Tzioufas, A.G.; Manousakis, M.N.; Kirou, K.A.; Crow, M.K.; Routsias, J.G. Elevated levels of soluble CD40 ligand (sCD40l) in serum of patients with systemic autoimmune diseases. J. Autoimmun. 2006, 26, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Duffau, P.; Seneschal, J.; Nicco, C.; Richez, C.; Lazaro, E.; Douchet, I.; Bordes, C.; Viallard, J.F.; Goulvestre, C.; Pellegrin, J.L.; et al. Platelet CD154 potentiates interferon-α secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci. Transl. Med. 2010, 2, 47ra63. [Google Scholar] [CrossRef] [PubMed]
- Kerkmann, M.; Rothenfusser, S.; Hornung, V.; Towarowski, A.; Wagner, M.; Sarris, A.; Giese, T.; Endres, S.; Hartmann, G. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J. Immunol. 2003, 170, 4465–4474. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Huang, Y.S.; Tang, M.; Lv, T.Y.; Hu, C.X.; Tan, Y.H.; Xu, Z.M.; Yin, Y.B. Microarray analysis of microRNA expression in peripheral blood cells of systemic lupus erythematosus patients. Lupus 2007, 16, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Zhang, Y.; Khan, D.; Heid, B.; Caudell, D.; Crasta, O.; Ahmed, S.A. Identification of a common lupus disease-associated microRNA expression pattern in three different murine models of lupus. PLoS ONE 2010, 5, e14302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, J.; Li, P.; Li, M.; Liu, Y.; Liang, S.; Gong, J. Knockdown of microRNA-155 in kupffer cells results in immunosuppressive effects and prolongs survival of mouse liver allografts. Transplantation 2014, 97, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Cekic, C.; Casella, C.R.; Sag, D.; Antignano, F.; Kolb, J.; Suttles, J.; Hughes, M.R.; Krystal, G.; Mitchell, T.C. Myd88-dependent ship1 regulates proinflammatory signaling pathways in dendritic cells after monophosphoryl lipid a stimulation of Tlr4. J. Immunol. 2011, 186, 3858–3865. [Google Scholar] [CrossRef] [PubMed]
- Lind, E.F.; Millar, D.G.; Dissanayake, D.; Savage, J.C.; Grimshaw, N.K.; Kerr, W.G.; Ohashi, P.S. Mir-155 upregulation in dendritic cells is sufficient to break tolerance in vivo by negatively regulating ship1. J. Immunol. 2015, 195, 4632–4640. [Google Scholar] [CrossRef] [PubMed]
- Divekar, A.A.; Dubey, S.; Gangalum, P.R.; Singh, R.R. Dicer insufficiency and microRNA-155 overexpression in lupus regulatory t cells: An apparent paradox in the setting of an inflammatory milieu. J. Immunol. 2011, 186, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Thai, T.H.; Patterson, H.C.; Pham, D.H.; Kis-Toth, K.; Kaminski, D.A.; Tsokos, G.C. Deletion of microRNA-155 reduces autoantibody responses and alleviates lupus-like disease in the Faslpr mouse. Proc. Natl. Acad. Sci. USA 2013, 110, 20194–20199. [Google Scholar] [CrossRef] [PubMed]
- O'Connell, R.M.; Kahn, D.; Gibson, W.S.; Round, J.L.; Scholz, R.L.; Chaudhuri, A.A.; Kahn, M.E.; Rao, D.S.; Baltimore, D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity 2010, 33, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, G.; Beynon, V.; Mittal, A.; Joller, N.; Weiner, H.L. Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis. J. Immunol. 2011, 187, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Bluml, S.; Bonelli, M.; Niederreiter, B.; Puchner, A.; Mayr, G.; Hayer, S.; Koenders, M.I.; van den Berg, W.B.; Smolen, J.; Redlich, K. Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheum. 2011, 63, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Liu, Y.J. Regulation of Tlr7/9 signaling in plasmacytoid dendritic cells. Protein Cell 2013, 4, 40–52. [Google Scholar] [CrossRef] [PubMed]







| Gene | Sequence | Accession No. | Tm | Pdt | Location | |
|---|---|---|---|---|---|---|
| β-actin | Fwd | TTG CTG ACA GGA TGC AGA AG | NM_007392.3 | 58.2 | 147 | 1039–1058 |
| Rev | TGA TCC ACA TCT GCT GGA AG | – | 57.3 | – | 1185–1166 | |
| Irf7 | Fwd | GAT CTT CAA GGC CTG GGC TGT GG | NM_016850.3 | 57.1 | 220 | 611–633 |
| Rev | TCC AAG CTC CCG GCT AAG TT | – | 55.0 | – | 830–811 | |
| Ifitm3 | Fwd | GAT CGG CTT CTG TCA GAA CTA | NM_025378.2 | 57.2 | 154 | 209–229 |
| Rev | TTC CGA TCC CTA GAC TTC ACG GA | – | 62.5 | – | 362–340 | |
| Tlr7 | Fwd | TGT TAC TAT TCC ATA CCT GGC CAC | NM_133211.4 | 60.1 | 179 | 2640–2663 |
| Rev | GGT GAC TTG TTG TCA TAA CTA CC | – | 57.1 | – | 2818–2796 | |
| Tlr9 | Fwd | CAA CAT GGT TCT CCG TCG AA | NM_031178.2 | 58.2 | 243 | 103–122 |
| Rev | TTG TGC AGG TGG TGG ATA CGG T | – | 64.2 | – | 345–324 | |
| Cd40 | Fwd | TTG TTG ACA GCG GTC CAT CT | NM_011611.2 | 59.6 | 154 | 112–131 |
| Rev | CTG AGT CAC ATG GGT GGC AT | – | 60.0 | – | 265–246 | |
| Ship1 | Fwd | CCA GGG CAA GAT GAG GGA GA | NM_001110193.2 | 60.98 | 195 | 2766–2785 |
| Rev | GGA CCT CGG TTG GCA ATG TA | – | 60.04 | – | 2960–2941 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Yim, L.Y.; Tam, R.C.Y.; Chan, A.; Lu, L.; Lau, C.S.; Chan, V.S.-F. MicroRNA-155 Mediates Augmented CD40 Expression in Bone Marrow Derived Plasmacytoid Dendritic Cells in Symptomatic Lupus-Prone NZB/W F1 Mice. Int. J. Mol. Sci. 2016, 17, 1282. https://doi.org/10.3390/ijms17081282
Yan S, Yim LY, Tam RCY, Chan A, Lu L, Lau CS, Chan VS-F. MicroRNA-155 Mediates Augmented CD40 Expression in Bone Marrow Derived Plasmacytoid Dendritic Cells in Symptomatic Lupus-Prone NZB/W F1 Mice. International Journal of Molecular Sciences. 2016; 17(8):1282. https://doi.org/10.3390/ijms17081282
Chicago/Turabian StyleYan, Sheng, Lok Yan Yim, Rachel Chun Yee Tam, Albert Chan, Liwei Lu, Chak Sing Lau, and Vera Sau-Fong Chan. 2016. "MicroRNA-155 Mediates Augmented CD40 Expression in Bone Marrow Derived Plasmacytoid Dendritic Cells in Symptomatic Lupus-Prone NZB/W F1 Mice" International Journal of Molecular Sciences 17, no. 8: 1282. https://doi.org/10.3390/ijms17081282
APA StyleYan, S., Yim, L. Y., Tam, R. C. Y., Chan, A., Lu, L., Lau, C. S., & Chan, V. S. -F. (2016). MicroRNA-155 Mediates Augmented CD40 Expression in Bone Marrow Derived Plasmacytoid Dendritic Cells in Symptomatic Lupus-Prone NZB/W F1 Mice. International Journal of Molecular Sciences, 17(8), 1282. https://doi.org/10.3390/ijms17081282