Development of a Patient-Derived Xenograft (PDX) of Breast Cancer Bone Metastasis in a Zebrafish Model
Abstract
:1. Introduction
2. Results
2.1. Patient History
2.2. Breast Cancer Cell Lines
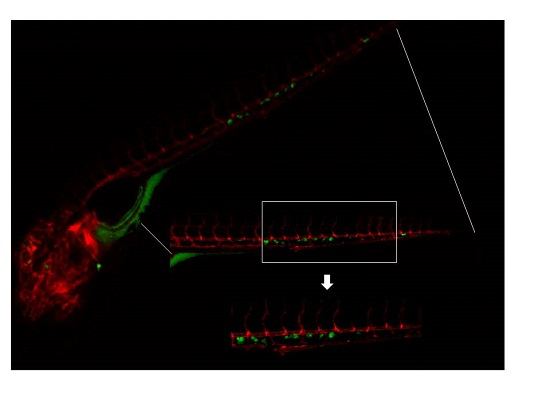
2.3. Invasive Phenotype of Primary Culture of Bone Metastasis in Zebrafish Model
2.4. Overexpression of Osteomimicry Markers by Primary Culture of Bone Metastasis
3. Discussion
4. Experimental Section
4.1. Cell Cultures
4.2. Isolation of Primary Bone Metastasis Cells
4.3. Immunocytochemistry
4.4. Prepapration of Cells for Implantation into Zebrafish (ZF) Embryos
4.5. In Vivo Experiments with the ZF Model
4.6. Quantitative Real-Time PCR (qPCR)
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BM | bone metastasis |
| CDH1 | cadherin-1 |
| CHT | caudal hematopoietic tissues |
| CTGF | connective tissue growth factor |
| CXCR4 | C-X-C chemokine receptor type 4 |
| dpf | days post fertilization |
| HSCs | hematopoietic stem cells |
| HPSE | heparanase |
| HPRT | hypoxanthine phosphoribosyltransferase 1 |
| IBSP | integrin binding sialoprotein |
| IL6 | interleukin 6 |
| LOX | lysyl oxidase |
| MMP-9 | matrix metallopeptidase 9 |
| JAG1 | jagged1 |
| OPG | osteoprotegerin |
| PBS | phosphate buffered saline |
| PDX | patient-derived material |
| PThrP | parathyroid hormone-related protein |
| RANK | receptor activator of nuclear factor κB |
| RANKL | receptor activator of nuclear factor κB ligand |
| TGFβ | transforming growth factor β |
| SPARC | secreted protein acidic and cysteine rich |
| TFF1 | trefoil factor 1 |
| Ct | threshold cycle |
| VEGFR1 | vascular endothelial growth factor receptor 1 |
| ZF | Zebrafish |
References
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Rüger, R. Molecular mechanisms of bone metastasis. Cancer Genom. Proteom. 2016, 13, 1–12. [Google Scholar]
- Guan, X. Cancer metastasis: Challenges and opportunitis. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Mercatali, L.; Amadori, D. A new emergency in oncology: Bone metastases in breast cancer patients. Oncol. Lett. 2013, 6, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the premetastatic niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Esposito, M.; Kang, Y. Bone metastasis and the metastatic niche. J. Mol. Med. 2015, 93, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Snaar-Jagalska, B.E. ZF-CANCER: Developing high-throughput bioassays for human cancers in zebrafish. Zebrafish 2009, 6, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Tulotta, C.; Stefanescu, C.; Beletkaia, E.; Bussmann, J.; Tarbashevich, K.; Schmidt, T.; Snaar-Jagalska, B.E. Inhibition of signaling between human CXCR4 and zebrafish ligands by the small molecule IT1t impairs the formation of triple-negative breast cancer early metastases in a zebrafish xenograft model. Dis. Model. Mech. 2016, 9, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Barriuso, J.; Nagaraju, R.; Hurlstone, A. Zebrafish: A new companion for translational research in oncology. Clin. Cancer Res. 2015, 21, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Holen, I.; Walker, M.; Nutter, F.; Fowles, A.; Evans, C.A.; Eaton, C.L.; Ottewell, P.D. Oestrogen receptor positive breast cancer metastasis to bone: Inhibition by targeting the bonemicroenvironment in vivo. Clin. Exp. Metastasis 2016, 33, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Reeves, K.J.; Brown, H.K.; Fowles, A.C.; Docherty, F.E.; Ottewell, P.D.; Croucher, P.I.; Holen, I.; Eaton, C.L. The frequency of osteolytic bone metastasis is determined by conditions of the soil, not the number of seeds; evidence from in vivo models of breast and prostate cancer. J. Exp. Clin. Cancer Res. 2015, 20, 34–124. [Google Scholar] [CrossRef] [PubMed]
- Ell, B.; Mercatali, L.; Ibrahim, T.; Campbell, N.; Schwarzenbach, H.; Pantel, K.; Amadori, D.; Kang, Y. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bonemetastasis. Cancer Cell 2013, 24, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Kang, Y. Efficient acquisition of dual metastasis organotropism to bone and lung through stable spontaneous fusion between MDA-MB-231 variants. Proc. Natl. Acad. Sci. USA 2009, 106, 9385–9390. [Google Scholar] [CrossRef] [PubMed]
- Weiss, F.U.; Marques, I.J.; Woltering, J.M.; Vlecken, D.H.; Aghdassi, A.; Partecke, L.I.; Heidecke, C.D.; Lerch, M.M.; Bagowski, C.P. Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology 2009, 137, 2136–2145. [Google Scholar] [CrossRef] [PubMed]
- Marques, I.J.; Weiss, F.U.; Vlecken, D.H.; Nitsche, C.; Bakkers, J.; Lagendijk, A.K.; Partecke, L.I.; Heidecke, C.D.; Lerch, M.M.; Bagowski, C.P. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer 2009, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Razaq, W. Bone targeted therapies for bone metastasis in breast cancer. J. Clin. Med. 2013, 2, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Zon, L.I. Zebrafish tumor assays: The state of transplantation. Zebrafish 2009, 6, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.M.; Seftor, E.A.; Bonde, G.; Cornell, R.A.; Hendrix, M.J. The fate of human malignant melanoma cells transplanted into zebrafish embryos: Assessment of migration and cell division in the absence of tumor formation. Dev. Dyn. 2005, 233, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Stoletov, K.; Montel, V.; Lester, R.D.; Gonias, S.L.; Klemke, R. High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc. Natl. Acad. Sci. USA 2007, 104, 17406–17411. [Google Scholar] [CrossRef] [PubMed]
- Haldi, M.; Ton, C.; Seng, W.L.; McGrath, P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 2006, 9, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Moore, J.C.; Ignatius, M.S.; Tenen, I.M.; Hayes, M.N.; Garcia, E.G.; Torres, Y.N.; Bourque, C.; He, S.; Blackburn, J.S.; et al. Imaging tumour cell heterogeneity following cell transplantation into optically clear immune-deficient zebrafish. Nat. Commun. 2016, 7, 10358. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xie, X.; Walker, S.; White, D.T.; Mumm, J.S.; Cowell, J.K. Evaluating human cancer cell metastasis in zebrafish. BMC Cancer 2013, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Murayama, E.; Kissa, K.; Zapata, A.; Mordelet, E.; Briolat, V.; Lin, H.-F.; Handin, R.I.; Herbomel, P. Tracing hematopoietic precursor migration to successive hematopoietic organs during Zebrafish development. Immunity 2006, 25, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Sacco, A.; Roccaro, A.M.; Ma, D.; Shi, J.; Mishima, Y.; Moschetta, M.; Chiarini, M.; Munshi, N.; Handin, R.I.; Ghobrial, I.M. Cancer cell dissemination and homing to the bone marrow in a zebrafish model. Cancer Res. 2016, 76, 463–471. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lamers, G.E.; Beenakker, J.W.; Cui, C.; Ghotra, V.P.; Danen, E.H.; Meijer, A.H.; Spaink, H.P.; Snaar-Jagalska, B.E. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J. Pathol. 2012, 227, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Kang, Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacol. Ther. 2014, 141, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.; Webber, M.; Benayahu, D.; Kleinman, H.K. Osteonectin promotes prostate cancer cell migration and invasion: A possible mechanism for metastasis to bone. Cancer Res. 1999, 59, 4453–4457. [Google Scholar] [PubMed]
- Li, S.; Zou, D.; Li, C.; Meng, H.; Sui, W.; Feng, S.; Cheng, T.; Zhai, Q.; Qiu, L. Targeting stem cell niche can protect hematopoietic stem cells from chemotherapy and G-CSF treatment. Stem Cell Res. Ther. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.G.; Calvi, L.M. Notch signaling in the malignant bone marrow microenvironment: Implications for a niche-based model of oncogenesis. Ann. N. Y. Acad. Sci. 2015, 1335, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Windrichova, J.; Fuchsova, R.; Kucera, R.; Topolcan, O.; Fiala, O.; Finek, J.; Slipkova, D.; Karlikova, M.; Svobodova, J. Testing of a novel cancer metastatic multiplex panel for the detection of bone-metastatic disease—A pilot study. Anticancer Res. 2016, 36, 1973–1978. [Google Scholar] [PubMed]
- Sethi, N.; Kang, Y. Notch signalling in cancer progression and bone metastasis. Br. J. Cancer 2011, 105, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.C.; Chung, L.W. RANK-mediated signaling network and cancer metastasis. Cancer Metastasis Rev. 2014, 33, 497–509. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercatali, L.; La Manna, F.; Groenewoud, A.; Casadei, R.; Recine, F.; Miserocchi, G.; Pieri, F.; Liverani, C.; Bongiovanni, A.; Spadazzi, C.; et al. Development of a Patient-Derived Xenograft (PDX) of Breast Cancer Bone Metastasis in a Zebrafish Model. Int. J. Mol. Sci. 2016, 17, 1375. https://doi.org/10.3390/ijms17081375
Mercatali L, La Manna F, Groenewoud A, Casadei R, Recine F, Miserocchi G, Pieri F, Liverani C, Bongiovanni A, Spadazzi C, et al. Development of a Patient-Derived Xenograft (PDX) of Breast Cancer Bone Metastasis in a Zebrafish Model. International Journal of Molecular Sciences. 2016; 17(8):1375. https://doi.org/10.3390/ijms17081375
Chicago/Turabian StyleMercatali, Laura, Federico La Manna, Arwin Groenewoud, Roberto Casadei, Federica Recine, Giacomo Miserocchi, Federica Pieri, Chiara Liverani, Alberto Bongiovanni, Chiara Spadazzi, and et al. 2016. "Development of a Patient-Derived Xenograft (PDX) of Breast Cancer Bone Metastasis in a Zebrafish Model" International Journal of Molecular Sciences 17, no. 8: 1375. https://doi.org/10.3390/ijms17081375
APA StyleMercatali, L., La Manna, F., Groenewoud, A., Casadei, R., Recine, F., Miserocchi, G., Pieri, F., Liverani, C., Bongiovanni, A., Spadazzi, C., De Vita, A., Van der Pluijm, G., Giorgini, A., Biagini, R., Amadori, D., Ibrahim, T., & Snaar-Jagalska, E. (2016). Development of a Patient-Derived Xenograft (PDX) of Breast Cancer Bone Metastasis in a Zebrafish Model. International Journal of Molecular Sciences, 17(8), 1375. https://doi.org/10.3390/ijms17081375