Bone Density as a Marker of Response to Radiotherapy in Bone Metastatic Lesions: A Review of the Published Data
Abstract
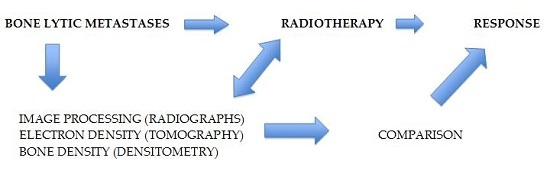
:1. Introduction
2. Search Procedure and Published Data
3. Discussion
4. Conclusions
Conflicts of Interest
Abbreviations
| AIS | Analgesic intake scale |
| BPS | Bone pain score |
| CI | Confidence Interval |
| CT | Computed tomography |
| DP | Pamidronate disodium |
| ECOG | Eastern Cooperative Oncology Group |
| EGLH | Energy gray value histogram |
| EORTC QLQ-C30 | European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C-30 |
| i.v | Intravenous |
| HU | Hounsfield Units |
| KPS | Karnofski Performance Status |
| MRI | Magnetic Resonance Image |
| MVGLH | Mean value gray level histogram |
| QOL | Quality of life |
| RED | Relative electron density |
| SD | Standard Deviation |
| UHPC | Urine hydroxyproline-creatine ratio |
References
- Li, S.; Peng, Y.; Weinhandl, E.D.; Blaes, A.H.; Cetin, K.; Chia, V.M.; Stryker, S.; Pinzone, J.J.; Acquavella, J.F.; Arneson, T.J. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin. Epidemiol. 2012, 4, 87–93. [Google Scholar] [PubMed]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12 (Suppl. S20), 6243–6249. [Google Scholar] [CrossRef] [PubMed]
- Yong, M.; Jensen, A.Ö.; Jacobsen, J.B.; Nørgaard, M.; Fryzek, J.P.; Sørensen, H.T. Survival in breast cancer patients with bone metastases and skeletal-related events: A population-based cohort study in Denmark (1999–2007). Breast Cancer Res. Treat. 2011, 129, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Lipton, A.; Cook, R.; Chen, Y.M.; Smith, M.; Coleman, R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 2007, 110, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, G.; Scagliotti, G. Management of bone metastases in cancer: A review. Crit. Rev. Oncol. Hematol. 2005, 56, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.; Zeng, L.; Salvo, N.; Dennis, K.; Tsao, M.; Lutz, S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin. Oncol. 2012, 24, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Wong, R.; Johnston, M.; Bezjak, A.; Whelan, T.; Cancer Care Ontario Practice Guidelines Initiative Supportive Care Group. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 594–605. [Google Scholar] [CrossRef]
- Roos, D.E.; Turner, S.L.; O’Brien, P.C.; Smith, J.G.; Spry, N.A.; Burmeister, B.H.; Hoskin, P.J.; Ball, D.L.; Trans-Tasman Radiation Oncology Group, TROG 96.05. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone matastases (Trans-Tasman Radiation Oncology Group, TROG 96.05). Radiother. Oncol. 2005, 75, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Foro Arnalot, P.; Fontanals, A.V.; Galcerán, J.C.; Lynd, F.; Latiesas, X.S.; de Dios, N.R.; Castillejo, A.R.; Bassols, M.L.; Galán, J.L.; Conejo, I.M.; et al. Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases. Radiother. Oncol. 2008, 89, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Yarnold, J.R. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: Randomized comparison with a multifraction schedule over 12 months of patient follow-up. On behalf of the Bone Pain Trial Working Party. Radiother. Oncol. 1999, 52, 111–121. [Google Scholar] [CrossRef]
- Steenland, E.; Leer, J.W.; van Houwelingen, H.; Post, W.J.; van den Hout, W.B.; Kievit, J.; de Haes, H.; Martijn, H.; Oei, B.; Vonk, E.; et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: A global analysis of the Dutch Bone Metastasis Study. Radiother. Oncol. 1999, 52, 101–109. [Google Scholar] [CrossRef]
- Hartsell, W.F.; Scott, C.B.; Bruner, D.W.; Scarantino, C.W.; Ivker, R.A.; Roach, M., 3rd; Suh, J.H.; Demas, W.F.; Movsas, B.; Petersen, I.A.; et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J. Natl. Cancer Inst. 2005, 97, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.S.; Bentzen, S.M.; Sandberg, E.; Gadeberg, C.C.; Timothy, A.R. Randomized trial of single dose versus fractionated palliative radiotherapy of bone metastases. Radiother. Oncol. 1998, 47, 233–240. [Google Scholar] [CrossRef]
- Kaasa, S.; Brenne, E.; Lund, J.A.; Fayers, P.; Falkmer, U.; Holmberg, M.; Lagerlund, M.; Bruland, O. Prospective randomized multicenter trial on single fraction radiotherapy (8 Gy × 1) versus multiple fractions (3 Gy × 10) in the treatment of painful bone metastases. Radiother. Oncol. 2006, 79, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Kouloulias, E.V.; Kouvaris, R.J.; Antypas, C.; Mystakidou, K.; Matsopoulos, G.; Uzunoglu, C.N.; Moulopoulos, A.; Vlahos, J.L. An intra-patient dose-escalation dose of disodium pamidronate plus radiotherapy versus radiotherapy alone for the treatment of osteolytic metastases. Monitoring of recalcification using image-processing techniques. Strahlenther. Oncol. 2003, 179, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, V.; Kalogeropoulou, C.; Giannopoulou, E.; Leotsinidis, M.; Tsota, I.; Kardamakis, D. A novel study investigating the therapeutic outcome of patients with lytic, mixed and sclerotic bone metastases treated with combined radiotherapy and ibandronate. Clin. Exp. Metastasis 2007, 24, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, V.; Kalogeropoulou, C.; Christopoulos, C.; Solomou, E.; Leotsinides, M.; Kardamakis, D. Combination ibandronate and radiotherapy for the treatment of bone metastases: Clinical evaluation and radiologic assessment. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Foerster, R.; Eisele, C.; Bruckner, T.; Bostel, T.; Schlampp, I.; Wolf, R.; Debus, J.; Rief, H. Bone density as a marker for local response to radiotherapy of spinal bone metastases in women with breast cancer: A retrospective analysis. Radiat. Oncol. 2015, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rief, H.; Petersen, L.C.; Omlor, G.; Akbar, M.; Bruckner, T.; Rieken, S.; Haefner, M.; Shlampp, I.; Debus, J.; Welzel, T. German Bone Research Group. The effect of resistance training during radiotherapy on spinal bone metastases in cancer patients—A randomized trial. Radiother. Oncol. 2014, 112, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.B.; Jacob, S.A.; Gebsky, V. Utility-adjusted analysis of the cost of palliative radiotherapy for bone metastases. Australas. Radiol. 2003, 47, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumors: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Kouloulias, V.; Antypas, C.; Dardoufas, C.; Vlahos, L. Evaluation of recalcification of bone metastases after radiotherapy and i.v. infusion of disodium pamidronate, using imaging processing techniques. Comparative assessment using measurements of the optical density of plain radiography. Phys. Med. 2001, 17, 17–24. [Google Scholar]
- Kouloulias, V.; Matsopoulos, G.; Kouvaris, J.; Dardoufas, C.; Bottomley, A.; Varela, M.; Uzunoglu, N.; Antypas, C.; Metafa, A.; Moulopoulos, A.; et al. Radiotherapy in conjunction with intravenous infusion of 180 mg of disodium pamidronate in management of osteolytic metastases from breast cancer: Clinical evaluation, biochemical markers, quality of life, and monitoring of recalcification using assessments of gray-level histogram in plain radiographs. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 143–157. [Google Scholar] [PubMed]
- Kouloulias, V.E.; Matsopoulos, G.; Kouvaris, J.R.; Antypas, C.; Uzunoglu, N.C.; Varela, M.N.; Metafa, A.; Sandilos, P.; Vlahos, L.J. Limitations of quantitative bone-mass measurements using assessments of first-order statistics of grey-level histograms in plain radiographs. Acta Radiol. 2004, 45, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J. Relative electron density calibration of CT scanners for radiotherapy treatment planning. Br. J. Radiol. 1999, 72, 781–786. [Google Scholar] [CrossRef] [PubMed]

| Study | Number of Patients | Evaluated Parameters |
|---|---|---|
| Kouloulias et al. [15] | 42 | MVGLH, EGLH, RED, BPS, AIS, ECOG status, UHPC |
| Vassiliou et al. [16] | 52 | Pain relief, opiod use, QOL, Karnofky Performance Status, bone density |
| Vassiliou et al. [17] | 45 | Pain relief, opioid use, physical phunctioning, QOL, bone density, MRI |
| Foerster et al. [18] | 115 | Bone density |
| Rief et al. [19] | 60 | Bone density, pathological fractures |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouloulias, V.; Liakouli, Z.; Zygogianni, A.; Mystakidou, K.; Kouvaris, J.R. Bone Density as a Marker of Response to Radiotherapy in Bone Metastatic Lesions: A Review of the Published Data. Int. J. Mol. Sci. 2016, 17, 1391. https://doi.org/10.3390/ijms17091391
Kouloulias V, Liakouli Z, Zygogianni A, Mystakidou K, Kouvaris JR. Bone Density as a Marker of Response to Radiotherapy in Bone Metastatic Lesions: A Review of the Published Data. International Journal of Molecular Sciences. 2016; 17(9):1391. https://doi.org/10.3390/ijms17091391
Chicago/Turabian StyleKouloulias, Vassilis, Zoi Liakouli, Anna Zygogianni, Kyriaki Mystakidou, and John R. Kouvaris. 2016. "Bone Density as a Marker of Response to Radiotherapy in Bone Metastatic Lesions: A Review of the Published Data" International Journal of Molecular Sciences 17, no. 9: 1391. https://doi.org/10.3390/ijms17091391
APA StyleKouloulias, V., Liakouli, Z., Zygogianni, A., Mystakidou, K., & Kouvaris, J. R. (2016). Bone Density as a Marker of Response to Radiotherapy in Bone Metastatic Lesions: A Review of the Published Data. International Journal of Molecular Sciences, 17(9), 1391. https://doi.org/10.3390/ijms17091391