Wnt/β-Catenin Signaling Mediated-UCH-L1 Expression in Podocytes of Diabetic Nephropathy
Abstract
:1. Introduction
2. Results
2.1. High Glucose Increased the Expression of UCH-L1 in Podocytes in Vitro
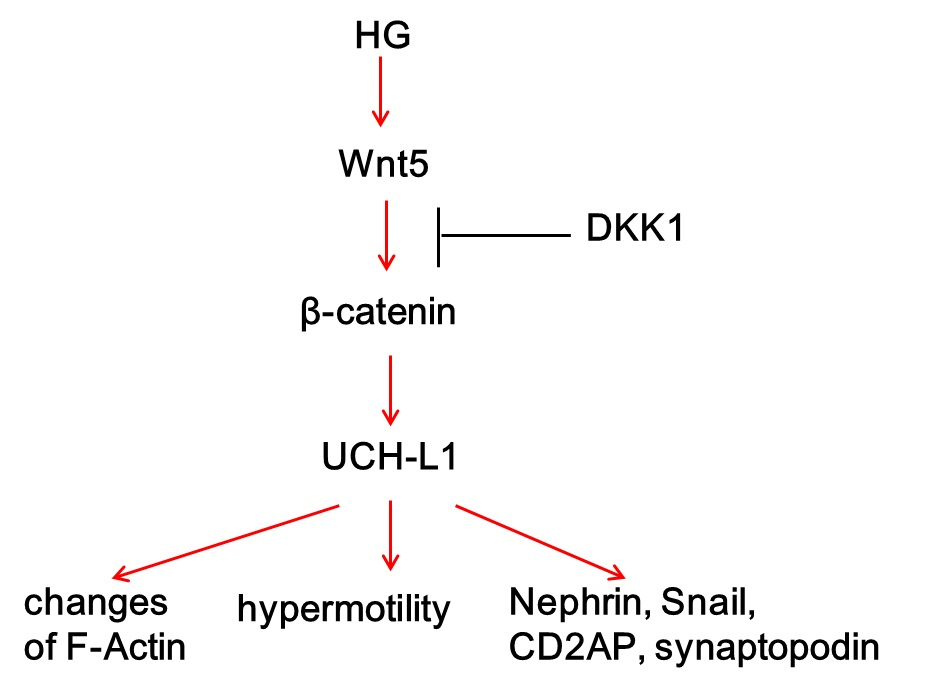
2.2. Activation of Wnt/β-Catenin Pathway Induced by High Glucose in Podocytes
2.3. Inhibition of Wnt/β-Catenin Pathway Downregulated High Glucose-Induced UCH-L1 Upregulation
2.4. UCH-L1 and β-Catenin Distribution in Podocytes of Diabetic Nephropathy Patients
2.5. UCH-L1 Overexpression Induced Changes of F-Actin and Hypermotility in Podocytes
2.6. UCH-L1 Overexpression Changed the Expression of Several Proteins in Podocytes
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Western Blot Analysis
4.3. Quantitative Real-Time RT-PCR
4.4. Immunofluorescence
4.5. Cell Migration Assay
4.6. F-Actin Staining
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Greka, A.; Mundel, P. Cell biology and pathology of podocytes. Annu. Rev. Physiol. 2012, 74, 299–323. [Google Scholar] [CrossRef] [PubMed]
- Covington, M.D.; Schnellmann, R.G. Chronic high glucose downregulates mitochondrial calpain 10 and contributes to renal cell death and diabetesinduced renal injury. Kidney Int. 2012, 81, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Amerik, A.Y.; Hochstrasser, M. Mechanism and function of deubiquitinating enzymes. Biochim. Biophys. Acta 2004, 1695, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, H. The role of the ubiquitin-proteasome system in kidney diseases. Clin. Exp. Nephrol. 2012, 16, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.O.; Barber, P.C.; Hamid, Q.A.; Power, B.F.; Dhillon, A.P.; Rode, J.; Day, I.N.; Thompson, R.J.; Polak, J.M. The immunolocalization of protein gene product 9.5 using rabbit polyclonal and mouse monoclonal antibodies. Br. J. Exp. Pathol. 1988, 69, 91–104. [Google Scholar] [PubMed]
- D’Andrea, V.; Malinovsky, L.; Berni, A.; Biancari, F.; Biassoni, L.; di Matteo, F.M.; Corbellini, L.; Falvo, L.; Santoni, F.; Spyrou, M.; et al. The immunolocalization of PGP 9.5 in normal human kidney and renal cell carcinoma. G Chir. 1997, 18, 521–524. [Google Scholar] [PubMed]
- Shirato, I.; Asanuma, K.; Takeda, Y.; Hayashi, K.; Tomino, Y. Protein gene product 9.5 is selectively localized in parietal epithelial cells of Bowman’s capsule in the rat kidney. J. Am. Soc. Nephrol. 2000, 11, 2381–2386. [Google Scholar] [PubMed]
- Liu, Y.; Wu, J.J.; Wu, H.J.; Wang, T.Z.; Gan, H.L.; Zhang, X.; Liu, Y.; Li, R.X.; Zhao, Z.H.; Chen, Q.; et al. UCH-L1 expression of podocytes in diseased glomeruli and in vitro. J. Pathol. 2009, 217, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Schwesinger, C.; Meyer, T.N.; Munster, S.; Klug, P.; Saleem, M.; Helmchen, U.; Stahl, R.A. A new role for the neuronal ubiquitin C-terminal hydrolase-L1 (UCH-L1) in podocyte process formation and podocyte injury in human glomerulopathies. J. Pathol. 2009, 217, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Schwesinger, C.; Meyer, T.N.; Sievert, H.; Hoxha, E.; Sachs, M.; Klupp, E.M.; Munster, S.; Balabanov, S.; Carrier, L.; Helmchen, U.; et al. Ubiquitin C-terminal hydrolase-L1 activity induces polyubiquitin accumulation in podocytes and increases proteinuria in rat membranous nephropathy. Am. J. Pathol. 2011, 178, 2044–2057. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Siragy, H.M. High glucose induces podocyte injury via enhanced (pro) renin receptor-Wnt-β-catenin-snail signaling pathway. PLoS ONE 2014, 9, e89233. [Google Scholar] [CrossRef] [PubMed]
- Diez-Sampedro, A.; Lenz, O.; Fornoni, A. Podocytopathy in diabetes: A metabolic and endocrine disorder. Am. J. Kidney Dis. 2011, 58, 637–646. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wang, M.; Yang, S.; Liu, F.; Sun, L. A glimpse of the pathogenetic mechanisms of Wnt/β-catenin signaling in diabetic nephropathy. BioMed Res. Int. 2013, 2013, 987064–987071. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, J.; Xu, P.; Liu, S.; Yang, Z. Wnt/β-catenin signalling pathway mediates high glucose induced cell injury through activation of TRPC6 in podocytes. Cell Prolif. 2013, 46, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.Y.; Wu, Y.Y.; Zhao, C.Y.; Chen, M.Y.; Yuan, Y.G.; Xing, C.Y.; Zhang, B. The Wnt/β-catenin signaling pathway participates in rhein ameliorating kidney injury in DN mice. Mol. Cell. Biochem. 2016, 411, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Gruenwald, A.; Suh, J.H.; Miner, J.H.; Barisoni-Thomas, L.; Taketo, M.M.; Faul, C.; Millar, S.E.; Holzman, L.B.; Susztak, K. Wnt/β-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J. Biol. Chem. 2011, 286, 26003–26015. [Google Scholar] [CrossRef] [PubMed]
- Bheda, A.; Yue, W.; Gullapalli, A.; Whitehurst, C.; Liu, R.; Pagano, J.S.; Shackelford, J. Positive reciprocal regulation of ubiquitin C-terminal hydrolase L1 and β-catenin/TCF signaling. PLoS ONE 2009, 4, e5955. [Google Scholar] [CrossRef] [PubMed]
- Semenov, M.V.; Tamai, K.; Brott, B.K.; Kuhl, M.; Sokol, S.; He, X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 2001, 11, 951–961. [Google Scholar] [CrossRef]
- Zhang, H.X.; Mao, X.; Sun, Y.; Hu, R.M.; Luo, W.L.; Zhao, Z.H.; Chen, Q.; Zhang, Z.G. NF-κB upregulates ubiquitin C-terminal hydrolase 1 in diseased podocytes in glomerulonephritis. Mol. Med. Rep. 2015, 2, 2893–2901. [Google Scholar]
- Zhang, H.X.; Sun, Y.; HU, R.M.; Luo, W.L.; Mao, X.; Zhao, Z.H.; Chen, Q.; Zhang, Z.G. The regulation of the UCH-L1 gene by transcription factor NF-κB in podocytes. Cell Signal. 2013, 25, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Ilatovskaya, D.V.; Levchenko, V.; Lowing, A.; Shuyskiy, L.S.; Palygin, O.; Staruschenko, A. Podocyte injury in diabetic nephropathy: Implications of angiotensin II-dependent activation of TRPC channels. Sci. Rep. 2015, 5, 17637–17647. [Google Scholar] [CrossRef] [PubMed]
- Pourghasem, M.; Shafi, H.; Babazadeh, Z. Histological changes of kidney in diabetic nephropathy. Caspian J. Intern. Med. 2015, 3, 120–127. [Google Scholar]
- Basseres, E.; Coppotelli, G.; Pfirrmann, T.; Andersen, J.B.; Masucci, M.; Frisan, T. The ubiquitin C-terminal hydrolase UCH-L1 promotes bacterial invasion by altering the dynamics of the actin cytoskeleton. Cell. Microbiol. 2010, 12, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Rolen, U.; Freda, E.; Xie, J.; Pfirrmann, T.; Frisan, T.; Masucci, M.G. The ubiquitin C-terminal hydrolase UCH-L1 regulates B-cell proliferation and integrin activation. J. Cell. Mol. Med. 2009, 13, 1666–1678. [Google Scholar] [CrossRef] [PubMed]
- Frisan, T.; Coppotelli, G.; Dryselius, R.; Masucci, M.G. Ubiquitin C-terminal hydrolase-L1 interacts with adhesion complexes and promotes cell migration, survival, and anchorage independent growth. FASEB J. 2012, 26, 5060–5070. [Google Scholar] [CrossRef] [PubMed]











| Mouse Gene | Primer Sequence 5′ to 3′ | |
|---|---|---|
| Forward | Reverse | |
| UCH-L1 | AAGCAGACCATCGGAAACTC | GCTCTATCTTCGGGGGACA |
| Wnt1 | TGACCTCTTTGGGTATTATCA | GAGACAAGGAGAATGTAGGGT |
| Wnt2 | CTCGGTGGAATCTGGCTCTG | CACATTGTCACACATCACCCT |
| Wnt5a | CAACTGGCAGGACTTTCTCAA | CATCTCCGATGCCGGAACT |
| β-catenin | ATGGAGCCGGACAGAAAAGC | CTTGCCACTCAGGGAAGGA |
| β-Actin | CATCCGTAAAGACCTCTATGCCAAC | ATGGAGCCACCGATCCACA |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Luo, W.; Sun, Y.; Qiao, Y.; Zhang, L.; Zhao, Z.; Lv, S. Wnt/β-Catenin Signaling Mediated-UCH-L1 Expression in Podocytes of Diabetic Nephropathy. Int. J. Mol. Sci. 2016, 17, 1404. https://doi.org/10.3390/ijms17091404
Zhang H, Luo W, Sun Y, Qiao Y, Zhang L, Zhao Z, Lv S. Wnt/β-Catenin Signaling Mediated-UCH-L1 Expression in Podocytes of Diabetic Nephropathy. International Journal of Molecular Sciences. 2016; 17(9):1404. https://doi.org/10.3390/ijms17091404
Chicago/Turabian StyleZhang, Hongxia, Weili Luo, Yonghong Sun, Yanchun Qiao, Liying Zhang, Zhilian Zhao, and Shijun Lv. 2016. "Wnt/β-Catenin Signaling Mediated-UCH-L1 Expression in Podocytes of Diabetic Nephropathy" International Journal of Molecular Sciences 17, no. 9: 1404. https://doi.org/10.3390/ijms17091404
APA StyleZhang, H., Luo, W., Sun, Y., Qiao, Y., Zhang, L., Zhao, Z., & Lv, S. (2016). Wnt/β-Catenin Signaling Mediated-UCH-L1 Expression in Podocytes of Diabetic Nephropathy. International Journal of Molecular Sciences, 17(9), 1404. https://doi.org/10.3390/ijms17091404