Experimental Dissection of Metalloproteinase Inhibition-Mediated and Toxic Effects of Phenanthroline on Zebrafish Development
Abstract
:1. Introduction
2. Results
2.1. Zebrafish Embryos Are More Sensitive to PhN than PhE
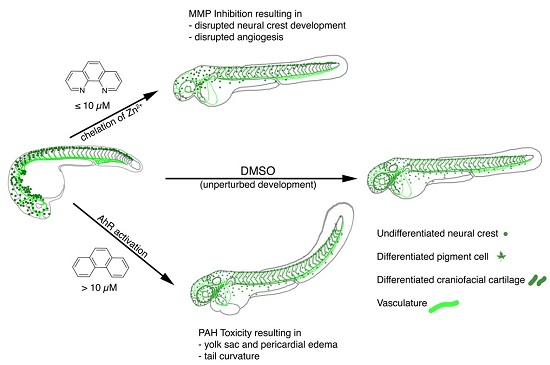
2.2. Loss of Pigmentation and Craniofacial Defects in PhN Treated Embryos Is due to Disruptions in Neural Crest Development
2.3. Loss of Intersomitic Circulation in PhN Treated Embryos Is due to Disruptions in Angiogenesis
2.4. Effects Common to PhE and PhN Can Be Rescued by Reducing Expression of the Aryl Hydrocarbon Receptor
3. Discussion
4. Materials and Methods
4.1. Zebrafish Husbandry
4.2. Phenanthrene (PhE) and Phenanthroline (PhN) Treatments
4.3. Scoring of Embryonic Defects
4.4. Immunofluorescence
4.5. Morpholino Injections
4.6. Imaging and Image Processing
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hadler-Olsen, E.; Fadnes, B.; Sylte, I.; Uhlin-Hansen, L.; Winberg, J.-O. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2011, 278, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Khokha, R.; Murthy, A.; Weiss, A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Apte, S.S.; Parks, W.C. Metalloproteinases: A parade of functions in matrix biology and an outlook for the future. Matrix Biol. 2015, 44, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Huxley-Jones, J.; Clarke, T.-K.; Beck, C.; Toubaris, G.; Robertson, D.L.; Boot-Handford, R.P. The evolution of the vertebrate metzincins; insights from Ciona intestinalis and Danio rerio. BMC Evol. Biol. 2007, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Cauwe, B.; Van den Steen, P.E.; Opdenakker, G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 113–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauwe, B.; Opdenakker, G. Intracellular substrate cleavage: A novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 351–423. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.P.; Patterson, N.L.; Fields, G.B.; Lindsey, M.L. The history of matrix metalloproteinases: Milestones, myths, and misperceptions. AJP Heart Circ. Physiol. 2012, 303, H919–H930. [Google Scholar] [CrossRef] [PubMed]
- Hellewell, A.L.; Adams, J.C. Insider trading: Extracellular matrix proteins and their non-canonical intracellular roles. BioEssays 2016, 38, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffney, J.; Solomonov, I.; Zehorai, E.; Sagi, I. Multilevel regulation of matrix metalloproteinases in tissue homeostasis indicates their molecular specificity in vivo. Matrix Biol. 2015, 44–46, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Small, C.; Crawford, B. Matrix metalloproteinases in neural development: A phylogenetically diverse perspective. Neural Regen. Res. 2016, 11, 357–362. [Google Scholar] [PubMed]
- Wyatt, R.A.; Keow, J.Y.; Harris, N.D.; Haché, C.A.; Li, D.H.; Crawford, B.D. The zebrafish embryo: A powerful model system for investigating matrix remodeling. Zebrafish 2009, 6, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Crawford, B.D.; Pilgrim, D.B. Ontogeny and regulation of matrix metalloproteinase activity in the zebrafish embryo by in vitro and in vivo zymography. Dev. Biol. 2005, 286, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Keow, J.Y.; Herrmann, K.M.; Crawford, B.D. Differential in vivo zymography: A method for observing matrix metalloproteinase activity in the zebrafish embryo. Matrix Biol. 2011, 30, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xie, X.; Walker, S.; White, D.; Mumm, J.; Cowell, J. Evaluating human cancer cell metastasis in zebrafish. BMC Cancer 2013, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Eckhardt, S.G. Development of matrix metalloproteinase inhibitors in cancer therapy. J. Natl. Cancer Inst. 2001, 93, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, Y.; Lee, H.M.; Hambardjieva, E.; Vrankova, K.M.; Golub, L.; Johnson, F. Design, synthesis and biological activity of new polyenolic inhibitors of matrix metalloproteinases: A focus on chemically-modified curcumins. Curr. Med. Chem. 2012, 19, 4348–4358. [Google Scholar] [CrossRef] [PubMed]
- Ramadoss, P.; Marcus, C.; Perdew, G.H. Role of the aryl hydrocarbon receptor in drug metabolism. Expert Opin. Drug Metab. Toxicol. 2005, 1, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Carney, S.A.; Peterson, R.E.; Heideman, W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Mol. Pharmacol. 2004, 66, 512–521. [Google Scholar] [PubMed]
- Dalton, T.P.; Puga, A.; Shertzer, H.G. Induction of cellular oxidative stress by aryl hydrocarbon receptor activation. Chemico-Biol. Interact. 2002, 141, 77–95. [Google Scholar] [CrossRef]
- Billiard, S.M.; Hahn, M.E.; Franks, D.G.; Peterson, R.E.; Bols, N.C.; Hodson, P.V. Binding of polycyclic aromatic hydrocarbons (PAHs) to teleost aryl hydrocarbon receptors (AHRs). Comp. Biochem. Physiol. 2002, 133, 55–68. [Google Scholar] [CrossRef]
- Reynaud, S.; Deschaux, P. The effects of polycyclic aromatic hydrocarbons on the immune system of fish: A review. Aquat. Toxicol. 2006, 77, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Logan, D.T. Perspective on ecotoxicology of PAHs to fish. Hum. Ecol. Risk Assess. 2007, 13, 302–316. [Google Scholar] [CrossRef]
- Hill, A.J.; Bello, S.M.; Prasch, A.L.; Peterson, R.E.; Heideman, W. Water permeability and TCDD-induced edema in zebrafish early-life stages. Toxicol. Sci. 2004, 78, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.N. The physiology and toxicology of salmonid eggs and larvae in relation to water quality criteria. Aquat. Toxicol. 2007, 81, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Teraoka, H.; Yamazaki, K.; Tsukiyama, S.; Imani, S.; Imagawa, T.; Stegeman, J.J.; Peterson, R.E.; Hiraga, T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: Local circulation failure in the dorsal midbrain is associated with increased apoptosis. Toxicol. Sci. 2002, 69, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; Stegeman, J.J.; Woodin, B.R.; Iwanaga, T.; Harano, R.; Peterson, R.E.; Hiraga, T.; Teraoka, H. Role of zebrafish cytochrome P450 CYP1C genes in the reduced mesencephalic vein blood flow caused by activation of AHR2. Toxicol. Appl. Pharmacol. 2011, 253, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Incardona, J.P.; Collier, T.K.; Scholz, N.L. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol. Appl. Pharmacol. 2004, 196, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P. Prespecification and plasticity: Shifting mechanisms of cell migration. Curr. Opin. Cell Biol. 2004, 16, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Vansaun, M.N.; Matrisian, L.M. Matrix metalloproteinases and cellular motility in development and disease. Birth Defects Res. 2006, 78, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Mayor, R. Molecular analysis of neural crest migration. Philos. Trans. R. Soc. 2008, 363, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Theveneau, E.; Mayor, R. Neural crest delamination and migration: From epithelium-to-mesenchyme transition to collective cell migration. Dev. Biol. 2012, 366, 34–54. [Google Scholar] [CrossRef] [PubMed]
- Leigh, N.R.; Schupp, M.O.; Li, K.; Padmanabhan, V.; Gastonguay, A.; Wang, L.; Chun, C.Z.; Wilkinson, G.A.; Ramchandran, R. Mmp17b is essential for proper neural crest cell migration in vivo. PLoS ONE 2013, 8, e76484. [Google Scholar] [CrossRef] [PubMed]
- Curran, K.; Raible, D.W.; Lister, J.A. Foxd3 controls melanophore specification in the zebrafish neural crest by regulation of Mitf. Dev. Biol. 2009, 332, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Carney, T.J.; Dutton, K.A.; Greenhill, E.; Delfino-Machín, M.; Dufourcq, P.; Blader, P.; Kelsh, R.N. A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development 2006, 133, 4619–4630. [Google Scholar] [CrossRef] [PubMed]
- Van Hinsbergh, V.W.M.; Koolwijk, P. Endothelial sprouting and angiogenesis: Matrix metalloproteinases in the lead. Cardiovasc. Res. 2008, 78, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Phng, L.K.; Gerhardt, H. Angiogenesis: A team effort coordinated by notch. Dev. Cell 2009, 16, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Isogai, S.; Lawson, N.D.; Torrealday, S.; Horiguchi, M.; Weinstein, B.M. Angiogenic network formation in the developing vertebrate trunk. Development 2003, 130, 5281–5290. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, M.L.; Guan, P.; Morris, R.J.; Fidock, M.D.; Rejzek, M.; Garcia-Morales, C.; Field, R.A.; Wheeler, G.N. A chemical genomic approach identifies matrix metalloproteinases as playing an essential and specific role in Xenopus melanophore migration. Chem. Biol. 2009, 16, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.; Bahudhanapati, H.; Wei, S. Extracellular metalloproteinases in neural crest development and craniofacial morphogenesis. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 544–560. [Google Scholar] [CrossRef] [PubMed]
- Crawford, B.D.; Po, M.D.; Saranyan, P.V.; Forsberg, D.; Schulz, R.; Pilgrim, D.B. Mmp25β facilitates elongation of sensory neurons during zebrafish development. Genesis 2014, 52, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Lagoutte, E.; Villeneuve, C.; Lafanechère, L.; Wells, C.M.; Jones, G.E.; Chavrier, P.; Rossé, C. LIMK regulates tumor-cell invasion and matrix degradation through tyrosine phosphorylation of MT1-MMP. Sci. Rep. 2016, 6, 24925. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Weinberg, R.A. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.R.; Blasky, A.J.; Britt, S.G.; Artinger, K.B. Riding the crest of the wave: Parallels between the neural crest and cancer in epithelial-to-mesenchymal transition and migration. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Maguire, L.H.; Thomas, A.R.; Goldstein, A.M. Tumors of the neural crest: Common themes in development and cancer. Dev. Dyn. 2015, 244, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Bedogni, B. The membrane tethered matrix metalloproteinase MT1-MMP at the forefront of melanoma cell invasion and metastasis. Pharmacol. Res. 2016, 111, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.T.; Murray, G.I. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2015, 237, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Monsonego-Ornan, E.; Kosonovsky, J.; Bar, A.; Roth, L.; Fraggi-Rankis, V.; Simsa, S.; Kohl, A.; Sela-Donenfeld, D. Matrix metalloproteinase 9/gelatinase B is required for neural crest cell migration. Dev. Biol. 2012, 364, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Zuasti, A.; Johnson, W.C.; Samaraweera, P.; Bagnara, J.T. Intrinsic pigment‐cell stimulating activity in the catfish integument. Pigment Cell Res. 1992, 5, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Wu, Y.; Yang, T.; Yang, K.; Wang, R.; Yang, J.; Guo, H. Wnt5a inhibits the proliferation and melanogenesis of melanocytes. Int. J. Med. Sci. 2013, 10, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Jung, H.; Lee, J.H.; Oh, H.Y.; Kim, O.B.; Han, I.O.; Oh, E.S. Keratinocyte-derived laminin-332 protein promotes melanin synthesis via regulation of tyrosine uptake. J. Biol. Chem. 2014, 289, 21751–21759. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Kawa, Y.; Tsai, R.K.; Shieh, J.H.; Lee, J.W.; Watabe, H.; Kawakami, T.; Soma, Y.; Tajima, S.; Mizoguchi, M. Melanocyte precursors express elastin binding protein and elastin-derived peptide (VGVAPG) stimulates their melanogenesis and dendrite formation. J. Dermatol. Sci. 2008, 51, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, Y.; Fan, R.; Chen, T.; Dong, C. MicroRNA-21a-5p functions on the regulation of melanogenesis by targeting Sox5 in mouse skin melanocytes. Int. J. Mol. Sci. 2016, 17, 959. [Google Scholar] [CrossRef] [PubMed]
- Paiva, K.B.S.; Granjeiro, J.M. Bone tissue remodeling and development: Focus on matrix metalloproteinase functions. Arch. Biochem. Biophys. 2014, 561, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Stetler-Stevenson, W.G. Matrix metalloproteinases in angiogenesis: A moving target for therapeutic intervention. J. Clin. Investig. 1999, 103, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jin, M.; Yang, F.; Zhu, J.; Xiao, Q.; Zhang, L. Matrix metalloproteinases: Inflammatory regulators of cell behaviors in vascular formation and remodeling. Med. Inflamm. 2013, 2013, 928315. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015, 44, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio); University of Oregon Press: Eugene, OR, USA, 1995. [Google Scholar]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]








© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellis, T.R.; Crawford, B.D. Experimental Dissection of Metalloproteinase Inhibition-Mediated and Toxic Effects of Phenanthroline on Zebrafish Development. Int. J. Mol. Sci. 2016, 17, 1503. https://doi.org/10.3390/ijms17091503
Ellis TR, Crawford BD. Experimental Dissection of Metalloproteinase Inhibition-Mediated and Toxic Effects of Phenanthroline on Zebrafish Development. International Journal of Molecular Sciences. 2016; 17(9):1503. https://doi.org/10.3390/ijms17091503
Chicago/Turabian StyleEllis, Tonya R., and Bryan D. Crawford. 2016. "Experimental Dissection of Metalloproteinase Inhibition-Mediated and Toxic Effects of Phenanthroline on Zebrafish Development" International Journal of Molecular Sciences 17, no. 9: 1503. https://doi.org/10.3390/ijms17091503
APA StyleEllis, T. R., & Crawford, B. D. (2016). Experimental Dissection of Metalloproteinase Inhibition-Mediated and Toxic Effects of Phenanthroline on Zebrafish Development. International Journal of Molecular Sciences, 17(9), 1503. https://doi.org/10.3390/ijms17091503