Xanthine Oxidase Inhibitors for Improving Renal Function in Chronic Kidney Disease Patients: An Updated Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
2.1. Search Results
2.2. Study Characteristics
2.3. Risk of Bias
2.4. Outcome Data
2.5. Effects of Xanthine Oxidase Inhibitors on Progression to ESKD
2.6. Effects of Xanthine Oxidase Inhibitors on Secondary Outcomes
2.6.1. Serum Creatinine
2.6.2. Renal Function
2.6.3. Proteinuria
2.6.4. Albuminuria
3. Discussion
4. Materials and Methods
4.1. Data Source and Search Strategy
4.2. Study Selection and Data Extraction
4.3. Data Analysis
4.4. Risk of Bias (Quality) Assessment
4.5. Summary of Findings and Quality of the Evidence
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Bolignano, D.; Pisano, A.; Coppolino, G. The Dark Side of Blocking RAS in Diabetic Patients with Incipient or Manifested Nephropathy. Exp. Clin. Endocrinol. Diabetes 2016, 124, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Ota, T.; Tamura, Y.; Chang, W.X.; Shibata, S.; Uchida, S. Time to target uric acid to retard CKD progression. Clin. Exp. Nephrol. 2017, 21, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sanchez-Lozada, L.G.; Kang, D.H.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transplant. 2013, 28, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Thurston, M.M.; Phillips, B.B.; Bourg, C.A. Safety and efficacy of allopurinol in chronic kidney disease. Ann. Pharmacother. 2013, 47, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Kabul, S.; Shepler, B. A review investigating the effect of allopurinol on the progression of kidney disease in hyperuricemic patients with chronic kidney disease. Clin. Ther. 2012, 34, 2293–2296. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Nakagawa, T.; Feng, L.; Watanabe, S.; Han, L.; Mazzali, M.; Truong, L.; Harris, R.; Johnson, R.J. A role for uric acid in the progression of renal disease. J. Am. Soc. Nephrol. 2002, 13, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Mazzali, M.; Hughes, J.; Kim, Y.G.; Jefferson, J.A.; Kang, D.H.; Gordon, K.L.; Lan, H.Y.; Kivlighn, S.; Johnson, R.J. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001, 38, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lozada, L.G.; Tapia, E.; Soto, V.; Avila-Casado, C.; Franco, M.; Wessale, J.L.; Zhao, L.; Johnson, R.J. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron Physiol. 2008, 108, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Bose, B.; Badve, S.V.; Hiremath, S.S.; Boudville, N.; Brown, F.G.; Cass, A.; de Zoysa, J.R.; Fassett, R.G.; Faull, R.; Harris, D.C.; et al. Effects of uric acid-lowering therapy on renal outcomes: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2014, 29, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Kanji, T.; Gandhi, M.; Clase, C.M.; Yang, R. Urate lowering therapy to improve renal outcomes in patients with chronic kidney disease: Systematic review and meta-analysis. BMC Nephrol. 2015, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Fleeman, N.; Pilkington, G.; Dundar, Y.; Dwan, K.; Boland, A.; Dickson, R.; Anijeet, H.; Kennedy, T.; Pyatt, J. Allopurinol for the treatment of chronic kidney disease: A systematic review. Health Technol. Assess. 2014, 18, 1–77. [Google Scholar] [CrossRef] [PubMed]
- Filiopoulos, V.; Hadjiyannakos, D.; Vlassopoulos, D. Febuxostat Renoprotection in CKD Patients With Asymptomatic Hyperuricemia. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2016, 67, 989–990. [Google Scholar] [CrossRef] [PubMed]
- Siu, Y.; Leung, K.; Tong, M.; Kwan, T. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am. J. Kidney Dis. 2006, 47, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Momeni, A.; Shahidi, S.; Seirafian, S.; Taheri, S.; Kheiri, S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran. J. Kidney Dis. 2010, 4, 128–132. [Google Scholar] [PubMed]
- Kao, M.; Ang, D.; Gandy, S.; Nadir, M.; Houston, J.; Lang, C.; Struthers, A. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J. Am. Soc. Nephrol. 2011, 22, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, W.; Jalal, D.; Li, Z.; Chen, W.; Mao, H.; Yang, Q.; Johnson, R.J.; Yu, X. Clinical outcome of hyperuricemia in IgA nephropathy: A retrospective cohort study and randomized controlled trial. Kidney Blood Press. Res. 2012, 35, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Ohno, I.; Nomura, S.; Hisatome, I.; Uchida, S.; Fujimori, S.; Yamamoto, T.; Hara, S. Effects of topiroxostat on the serum urate levels and urinary albumin excretion in hyperuricemic stage 3 chronic kidney disease patients with or without gout. Clin. Exp. Nephrol. 2014, 18, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Seo, Y.I.; Song, Y.W. Four-week effects of allopurinol and febuxostat treatments on blood pressure and serum creatinine level in gouty men. J. Korean Med. Sci. 2014, 29, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Sezer, S.; Karakan, S.; Atesagaoglu, B.; Acar, F.N. Allopurinol reduces cardiovascular risks and improves renal function in pre-dialysis chronic kidney disease patients with hyperuricemia. Saudi J. Kidney Dis. Transplant. 2014, 25, 316–320. [Google Scholar]
- Goicoechea, M.; Garcia de Vinuesa, S.; Verdalles, U.; Verde, E.; Macias, N.; Santos, A.; Perez de Jose, A.; Cedeno, S.; Linares, T.; Luno, J. Allopurinol and progression of CKD and cardiovascular events: Long-Term follow-up of a randomized clinical trial. Am. J. Kidney Dis. 2015, 65, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, M.; de Vinuesa, S.G.; Verdalles, U.; Ruiz-Caro, C.; Ampuero, J.; Rincon, A.; Arroyo, D.; Luno, J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin. J. Am. Soc. Nephrol. 2010, 5, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Bayram, D.; Tuqrul, S.M.; Inal, S.; Altunta, A.; Kidir, V.; Orhan, H. The effects of allopurinol on metabolic acidosis and endothelial functions in chronic kidney disease patients. Clin. Exp. Nephrol. 2015, 19, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.; Ivanova, M. Febuxostat improves GFR and BP in non-diabetic adults with CKD 2–3: 4 years follow-up. Nephrol. Dial. Transplant. 2015, 30, iii486–iii487. [Google Scholar]
- Sircar, D.; Chatterjee, S.; Waikhom, R.; Golay, V.; Raychaudhury, A.; Chatterjee, S.; Pandey, R. Efficacy of Febuxostat for Slowing the GFR Decline in Patients With CKD and Asymptomatic Hyperuricemia: A 6-Month, Double-Blind, Randomized, Placebo-Controlled Trial. Am. J. Kidney Dis. 2015, 66, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Nakayama, M.; Kanno, M.; Kimura, H.; Watanabe, K.; Tani, Y.; Hayashi, Y.; Asahi, K.; Terawaki, H.; Watanabe, T. Renoprotective effects of febuxostat in hyperuricemic patients with chronic kidney disease: A parallel-group, randomized, controlled trial. Clin. Exp. Nephrol. 2015, 19, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Beddhu, S.; Filipowicz, R.; Wang, B.; Wei, G.; Chen, X.; Roy, A.C.; DuVall, S.L.; Farrukh, H.; Habib, A.N.; Bjordahl, T.; et al. A Randomized Controlled Trial of the Effects of Febuxostat Therapy on Adipokines and Markers of Kidney Fibrosis in Asymptomatic Hyperuricemic Patients With Diabetic Nephropathy. Can. J. Kidney Health Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.; Whelton, A.; Becker, M.A.; MacDonald, P.; Hunt, B.; Gunawardhana, L. Impact of Febuxostat on Renal Function in Gout Patients With Moderate-to-Severe Renal Impairment. Arthritis Rheumatol. 2016, 68, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Jalal, D.I.; Chonchol, M.; Chen, W.; Targher, G. Uric acid as a target of therapy in CKD. Am. J. Kidney Dis. 2013, 61, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Yelken, B.; Caliskan, Y.; Gorgulu, N.; Altun, I.; Yilmaz, A.; Yazici, H.; Oflaz, H.; Yildiz, A. Reduction of uric acid levels with allopurinol treatment improves endothelial function in patients with chronic kidney disease. Clin. Nephrol. 2012, 77, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lozada, L.G.; Tapia, E.; Santamaria, J.; Avila-Casado, C.; Soto, V.; Nepomuceno, T.; Rodriguez-Iturbe, B.; Johnson, R.J.; Herrera-Acosta, J. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005, 67, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Santos, A.B.; Neogi, T. Management of Gout and Hyperuricemia in CKD. Am. J. Kidney Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Pisano, A.; D’Arrigo, G. Xanthine oxidase inhibitors for improving renal damage in CKD patients. Available online: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42017067881 (accessed on 31 May 2017).
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Study, Year (Ref) | Study Population | Population Characteristics | Duration | Intervention | Comparator | Outcome(s) | Results | Notes |
|---|---|---|---|---|---|---|---|---|
| Siu et al., 2006 [13] | -Hyperuricemic, mild to moderate CKD patients -Exclusion criteria: history of gouty arthritis, renal stones and advanced CKD, use of Allopurinol or Azathioprine, Allopurinol hypersensitivity, pregnancy or lactation | -N = 51 -Age (yr) = ~48.2 -Weight (kg) = ~68 -DM (%) = ~25.5 -Hypertension (%) = ~78.5 -SBP (mmHg) = ~136.5 -DBP (mmHg) = ~75 -Uric acid (mg/dL) = ~9.83 -SCr (mg/dL) = ~1.75 -Proteinuria (g/d) = ~2.39 | 12 months | Allopurinol, 100–200 mg/day (N = 25) | Standard therapy (N = 26) | SCr (mg/dL) | No difference between groups | -Open label -Allopurinol dose was adjusted according to baseline renal function -Antihypertensive, lipid-lowering and steroid drugs were continued during the study -One patient in the Allopurinol group withdrew due to urticarial skin rash; two lost to follow-up in control group -Per-protocol analysis performed |
| Proteinuria (g/day) | No difference between groups | |||||||
| Need for dialysis | One patient in Allopurinol (1/25) and control group (1/26), respectively | |||||||
| Momeni et al., 2010 [14] | -Type 2 diabetic patients with nephropathy (proteinuria ≥ 500 mg/d, SCr < 3 mg/dL) -Exclusion criteria: history of Allopurinol hypersensitivity or past use of Allopurinol for other reasons, SCr > 3 mg/dL or GFR < 25 mL/min, systemic diseases or other causes of proteinuria | -N = 40 -Men (%) = 45 -Age (yr) = 57.7 ± 10.5 -Weight (kg) = ~75.4 -BMI (kg/m2) = ~27.8 -DM duration (yr) = 12.6 ± 6.7 -SBP (mmHg) = ~146.5 -DBP (mmHg) = ~87.2 -Uric acid (mg/dL) = ~6.2 -SCr (mg/dL) = ~1.4 -Proteinuria (mg/d) = ~1714.5 -Urine Cr (mg/d) = ~1064.5 | 4 months | Allopurinol, 100 mg/day (N = 20) | Placebo (N = 20) | SCr (mg/dL) | No difference between groups | -Double blind -Patients continued their concomitant treatment |
| Proteinuria (mg/day) | -End of treatment, 1011 ± 767 vs. 1609 ± 1071 in allopurinol vs. placebo group (p = 0.049) | |||||||
| Kao et al., 2011 [15] | -Stage 3 CKD patients with LVH -Exclusion criteria: active gout, LVF with EF < 45%, severe hepatic disease, use of Warfarin, Theophyllin, Allopurinol, Chlorpropamide, immunosuppressive therapy, metastatic malignancy, pregnancy | -N = 53 -Men (%) = ~58 -Age (yr) = ~72.2 -SBP (mmHg) = ~142 -DBP (mmHg) = ~72.5 -Uric acid (mmol/L) = ~0.43 -eGFR (mL/min/1.73 m2) = ~45 -UPCR (mg/mmol) = ~37.5 | 9 months | Allopurinol, 300 mg/day (N = 27) | Placebo (N = 26) | eGFR (mL/min/1.73 m2) | No difference between groups | -Double blind -Patients continued their concomitant treatment -3/14 patients withdrew due to rash and arthralgia in Allopurinol group -Per-protocol analysis performed |
| UPCR (mg/mmol) | No difference between groups | |||||||
| Shi et al., 2012 [16] | -Hyperuricemic IgAN patients -Exclusion criteria: active gout, prednisone or immunosuppressive use within the preceding 2 months, ACEIs and/or ARBs use, Allopurinol intolerance, pregnancy | -N = 40 -Men (%) = ~55 -Age (yr) = ~40 -SBP (mmHg) = ~140 -DBP (mmHg) = ~87.7 -Hypertension (%) = ~45 -Uric acid (mg/dL) = ~7.9 -SCr (mg/dL) = ~1.35 -eGFR (mL/min) = ~66.5 -UPCR (mg/g) = ~898 | 6 months | Allopurinol, 100–300 mg/day (N = 21) | Standard therapy (N = 19) | eGFR (mL/min/1.73 m2) | No difference between groups | -Open label -Three patients in the Allopurinol and two patients in control group discontinued the study; no patients were lost to follow-up -ITT analysis performed |
| UPCR (mg/g) | No difference between groups | |||||||
| Hosoya et al., 2014 * [17] Hara et al., 2015 (post-hoc) Jomori et al., 2015 (post-hoc) | -Hyperuricemic stage 3 CKD patients with or without gout -Exclusion criteria: gouty arthritis within 2 weeks before the study, nephrotic syndrome, nephrolithiasis or urolithiasis, hyperuricemia secondary to cancer or other diseases, HbA1c ≥ 8%, severe hypertension, hepatic dysfunction, cancer, pregnancy, breastfeeding, serious heart disease | -N = 122 -Men (%) = ~54.5 -Age (yr) = ~63.5 -BMI (kg/m2) = ~25.6 -Uric acid (µmol/L) = ~503.8 -DM (%) = ~21.5 -Diabetic nephropathy (%) = ~16.5 -SBP (mmHg) = ~135 -DBP (mmHg) = ~84.5 -eGFR (mL/min/1.73 m2) = ~49.2 -UACR (mg/g) = ~35.8 | 22 weeks | Topiroxostat, 160 mg/day (N = 62) | Placebo (N = 60) | eGFR (mL/min/1.73 m2) | -No difference between groups -No difference between groups when stratifying for DM nephropathy and nephrosclerosis | -Double blind -Topiroxostat and placebo were administered orally for 2 weeks at an initial dose of 40 mg/day, followed by an increase to 80 mg/day for 4 weeks, to 120 mg/day for 8 weeks, and to 160 mg/day for other 8 weeks -Six and five patients, in the Topiroxostat and placebo group, respectively, withdrew from the study due to AEs -ITT analysis performed |
| UACR (%) | -Mean percent change -33 (95% CI, −45.0, −20.0) vs. −6 (95% CI, −22.0, 14.0) in Topiroxostat vs. placebo group (p = 0.009) -Mean percent change -33.8 vs. +9 (p = 0.059) and −44.8 vs. +3.4 (p = 0.022), in Topiroxostat vs. placebo group when stratifying for DM nephropathy and nephrosclerosis, respectively | |||||||
| Kim et al., 2014 [18] | -Gouty patients with early renal function impairment -Exclusion criteria: SCr > 1.5 mg/dL, use of thiazide diuretics or medications containing Aspirin or other salicylates, active liver disease and alcohol intake > 14 drinks/week | -N =179 -Men (%) =100 -Age (yr) =~50 -BMI (kg/m2) =~25.9 -SBP (mmHg) =~129.7 -DBP (mmHg) =~82.1 -SCr (mg/dL) =~1.2 -eGFR(mL/min/1.73 m2) = ~68.6 | 1 month | Febuxostat, 40 mg/day (N = 35) Febuxostat, 80 mg/day (N = 35) Febuxostat, 120 mg/day (N = 36) Allopurinol, 300 mg/day (N = 36) | Placebo (N = 37) | SCr (mg/dL) | -End of treatment, 1.19 ± 0.10 vs. 1.23 ± 0.06 in the combined Febuxostat group (N = 106) vs. placebo (p = 0.007) -No difference between Allopurinol and placebo | -Double blind -Seven patients (placebo = 1, Febuxostat 80 mg/d = 1, Febuxostat 120 mg/d = 2, Allopurinol = 2) missed a follow-up or withdrew prematurely after week 2 -Missing data were analysed by applying the last-observation-carried-forward method |
| eGFR (mL/min/1.73 m2) | -End of treatment, 69.96 ± 4.63 vs. 68.13 ± 4.62 in the combined Febuxostat group (N = 106) vs. placebo (p = 0.03) -No difference between Allopurinol and placebo group | |||||||
| Sezer et al., 2014 [19] | -Stage 3–4 CKD patients -Exclusion criteria: history of Allopurinol intolerance, ongoing Allopurinol treatment, active infections or inflammatory diseases, chronic liver disease and ongoing immunosuppressive therapy | -N = 96 -Men (%) = 57 -Age (yr) = 65.3 ± 12.4 -eGFR(mL/min/1.73 m2) = ~45.8 | 12 months | Allopurinol, 1.5 ± 0.8 mg/kg/d (N = 49) | Standard therapy (N = 47) | eGFR (mL/min/1.73 m2) | -End of treatment, mean change 3.3 ± 1.2 vs. −1.3 ± 0.6 in Allopurinol vs. control group (p = 0.04) | -Open label -No hematologic alterations or serious adverse events in relation to Allopurinol treatment |
| Goicoechea et al., 2015 * [20] Goicoechea et al., 2010 [21] | -Moderate CKD patients (eGFR < 60 mL/min/1.73 m2) -Exclusion criteria: history of hypersensitivity or past use of Allopurinol, active infections or inflammatory diseases, HIV infection, chronic hepatopathy and use of immunosuppressive therapy | -N = 107 -Age (yr) = ~71.7 -SBP (mmHg) = 147 ± 20 -DBP (mmHg) = 77 ± 11 -Uric acid (mg/dL) = ~7.6 -SCr (mg/dL) = ~1.8 -eGFR(mL/min/1.73 m2) = ~40 -Urinary albumin (mg/day) = ~36 (median) | 84 months | Allopurinol, 100 mg/day (N = 56) | Standard therapy (N = 51) | eGFR (mL/min/1.73 m2) | -End of treatment, 34.1 ± 12.9 vs. 26.2 ± 17.4 in Allopurinol vs. control group | -Single blind -Antihypertensive, lipid-lowering and antiplatelet drugs were continued during the study period -Two patients in Allopurinol group withdrew because of gastrointestinal symptoms -Nine patients in the control and 4 in the Allopurinol group were lost to follow-up -ITT analyses performed |
| Need for dialysis | 7/57 pts in Allopurinol and 13/56 in control group, respectively | |||||||
| eGFR decrease ≥ 50% or SCr doubling | 2/57 pts in Allopurinol and 11/56 in control group, respectively | |||||||
| Bayram et al., 2015 [22] | -Hyperuricemic (uric acid > 5.5 mg/dL) stage 2–4 CKD patients -Exclusion criteria: dialysis, hyperuricemia due to malignancy, peripheral arterial disease, gouty arthritis or history of Allopurinol intolerance, ongoing Allopurinol treatment, active infections or inflammatory diseases | -N = 60 -Men (%) = ~46.7 -Age (yr) = ~57.4 -BMI (kg/m2) = ~26.8 -SBP (mmHg) = ~133.6 -DBP (mmHg) = ~77.5 -DM (%) = ~57 -Hypertension (%) = 63.3 -Uric acid (mg/dL) = ~7.8 -SCr (mg/dL) = ~2.1 -CrCl (mL/min) = ~49.6 -Proteinuria (mg/d) = ~2136 | 3 months | Allopurinol, 300 mg/day (N = 30) | Standard therapy (N = 30) | eGFR (mL/min) | -Significant increase (43.4 ± 20.1 to 51.4 ± 24.9) in the Allopurinol group (p = 0.011) -No change in the control group | -Open label -Antihypertensive drugs, lipid-lowering agents and antiplatelet drugs were continued during the study -No adverse effects related to Allopurinol treatment |
| Proteinuria (mg/day) | No significant change in the Allopurinol or control group | |||||||
| Ivanov and Ivanova, 2015 [23] | -Non-diabetic stage 2–3 CKD patients with mild hypertension and no history of gout | -N = 56 -eGFR (mL/min) = 54 ± 3 | 14 months | Allopurinol, 300 mg/day (N = 20) Febuxostat, 80 mg/day (N = 16) | Standard therapy (N = 20) | eGFR (mL/min) | -End of treatment, increase in Febuxostat (+14 ± 3) vs. control group (p < 0.01) | -Open label |
| Urinary albumin (mg/day) | -End of treatment, decrease in Febuxostat (−138 ± 22) vs. control group (p < 0.01) | |||||||
| Sircar et al., 2015 [24] | -Stage 3–4 CKD patients with asymptomatic hyperuricemia (uric acid ≥ 7 mg/dL) -Exclusion criteria: medication (excluding diuretics) or conditions that may increase uric acid levels such as disorders of primary uric acid metabolism. Autosomal dominant polycystic kidney disease, pregnancy, lactation and symptomatic hyperuricemia | -N = 108 -Men (%) = ~70.5 -Age (yr) = ~57.3 -stage 3 CKD (%) = ~47 -stage 4 CKD (%) = ~54 -SBP (mmHg) = ~144 -DBP (mmHg) = ~82.9 -DM (%) = ~37.5 -Hypertension (%) = ~98 -Uric acid (mg/dL) = ~8.6 -SCr (mg/dL) = ~2.2 -eGFR(mL/min/1.73 m2) = ~32 | 6 months | Febuxostat, 40 mg/day (N = 54) | Placebo (N = 54) | eGFR (mL/min/1.73 m2) | End of treatment, 34.7 ± 18.1 vs. 28.2 ± 11.5 in Febuxostat vs. placebo group (p = 0.05) | -Double blind -Both groups received antihypertensive agents, including ACEIs or ARBs or diuretics -About 10% of the randomly assigned population withdrew -A modified ITT analysis was performed for efficacy and safety data (N = 98) -Two patients in Febuxostat had mild diarrhoea |
| eGFR decrease ≥10% | 38% vs. 40% in Febuxostat vs. placebo group (p = 0.004) | |||||||
| Tanaka et al., 2015 [25] | -Hyperuricemic (uric acid ≥ 7.0 mg/dL) stage 3 CKD patients -Exclusion criteria: acute/chronic inflammatory disease and/or malignancy, active gout, severe CV/respiratory/digestive disease within 6 months before study entry, pregnancy, medication with Febuxostat and/or Benzbromarone within 3 months before, immunosuppressive therapy | -N = 45 -Men (%) = ~87.5 -Age (yr) = ~68 -BMI (kg/m2) = ~25 -SBP (mmHg) = ~129 -DBP (mmHg) = ~78 -Diabetic nephropathy (%) = ~8 -Hypertension (%) = ~42 -Uric acid (mg/dL) = ~8.0 -SCr (mg/dL) = ~1.3 -eGFR(mL/min/1.73 m2) = ~44.6 -UPCR (g/g) = ~0.67 -UACR (mg/g) = ~78 | 3 months | Febuxostat, 40 mg/day (N = 25) | Standard therapy (N = 20) | SCr (mg/dL) | -No difference between groups | -Open label -Febuxostat was administered at an initial dose of 10 mg/d and up-titrated to 40 mg -2 patients in Febuxostat group withdrew due to rash and hypotension. -One patient in the control group and 2 patients in the Febuxostat group were lost to follow-up -21 patients in the Febuxostat and 19 in the control group were analysed after follow-up |
| eGFR (mL/min/1.73 m2) | -End of treatment, mean change −1.3 ± 4.0 vs. −0.4 ± 5.8 in Febuxostat vs. control group (p = 0.59) | |||||||
| UPCR (g/g) | End of treatment, mean change −0.36 ± 0.66 vs. 0.07 ± 0.38 in Febuxostat vs. control group (p = 0.018) | |||||||
| UACR (mg/g) | End of treatment, median change -25.3 (−357.0, 4.8) vs. +5.2 (−71.4, 105.5) in Febuxostat vs. control group (p = 0.035) | |||||||
| Beddhu et al., 2016 [26] | -Overweight or obese adults with hyperuricemia and type 2 diabetic nephropathy -Exclusion criteria: history of gout, concurrent use of Azathioprine, Mercaptopurine, Theophylline, Allopurinol or Warfarin, recent antibiotic therapy, pregnancy, active malignancy, active AIDS, chronic lung disease | -N = 80 -Men (%) = 65 -Age (yr) = 68 ± 10 -BMI (kg/m2) = 34.6 ± 6.8 -SBP (mmHg) = 127 ± 17 -DBP (mmHg) = 70 ± 12 -Hypertension (%) = 77.5 -Uric acid (μmol/L) = 426 ± 83 -eGFR(mL/min/1.73 m2) = 53.5 ± 17.2 -UACR (mg/mmol) = ~2.19 (median) | 6 months | Febuxostat, 80 mg/day (N = 40) | Placebo (N = 40) | eGFR (mL/min/1.73 m2) | No difference between groups | -Double blind -One patient in the placebo and 3 in the Febuxostat group withdrew from the study -ITT analysis performed |
| UACR (mg/mmol) | -End of treatment, median 1.07 (IQR 0.46, 6.99) vs. 1.15 (IQR 0.42, 7.10) in Febuxostat vs. placebo | |||||||
| Saag et al., 2016 [27] | -Hyperuricemic, gouty patients with moderate-to-severe CKD -Exclusion criteria: secondary hyperuricemia, xanthinuria, tophaceous gout, use of Aspirin >325 mg/day within 35 days prior to randomization, Allopurinol, Febuxostat or Colchicine hypersensitivity, CV disease, dialysis, liver disease, alcoholism | -N = 96 -Men (%) = 80.2 -Age (yr) = 65.7 ± 10.57 -BMI (kg/m2) = 33.4 ± 6.67 -Hypertension (%) =95.8 -DM (%) = 44.8 -Uric acid (mg/dL) = 10.5 ± 1.7 | 12 months | Febuxostat, 30 mg/twice daily (N = 32) Febuxostat, 40/80 mg/day (N = 32) | Placebo (N = 32) | SCr (mg/dL) | No difference between Febuxostat groups and the placebo | -Double blind -At study screening, any urate-lowering therapies were discontinued -SCr levels of ≥1.5 mg/dL occurred in 41% of patients receiving 30 mg Febuxostat, 50% of patients receiving 40/80 mg Febuxostat and 53% of patients receiving placebo -Efficacy and safety analyses performed by the last-observation-carried-forward method |
| eGFR (mL/min/1.73 m2) | No difference between Febuxostat groups and the placebo |
| Study, Year (Ref) | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessors | Incomplete Outcome Data | Selective Reporting | Other Sources of Bias |
|---|---|---|---|---|---|---|---|
| Siu et al., 2006 [13] | Low risk (randomization performed using a computer-generated list) | Unclear (not stated) | High Risk (open label) | Unclear (not stated) | Low risk (3 drop-outs; per-protocol analysis performed) | Low risk | None known |
| Momeni et al., 2010 [14] | Unclear (not stated) | Unclear (not stated) | Low Risk (double blind) | Unclear (not stated) | Unclear (not stated) | Low risk | None known |
| Kao et al., 2011 [15] | Unclear (not stated) | Unclear (not stated) | Low Risk (double blind) | Unclear (not stated) | High risk (overall 14 drop-outs; 15% vs. 25% in intervention vs. control. Per-protocol analysis performed) | Low risk | None known |
| Shi et al., 2012 [16] | Low risk (“randomization performed using a computer-generated random allocation sequence table”) | Low risk (“allocation was concealed by enclosing assignments in sequentially numbered, opaque-closed envelopes”) | High Risk (open label) | Unclear (not stated) | Low risk (5 drop-outs; ITT analysis performed) | Low risk | None known |
| Hosoya et al., 2014 * [17] Hara et al., 2015 Jomori et al., 2015 | Unclear (not stated) | Unclear (not stated) | Low Risk (double blind) | Unclear (not stated) | Low risk (11 drop-outs; ITT analysis performed) | Low risk | High risk of funding bias (study was funded by Sanwa Kagaku Kenkyusho Co., Ltd. (SKK) |
| Kim et al., 2014 [18] | Unclear (not stated) | Unclear (not stated) | Low Risk (double blind) | Unclear (not stated) | Low risk (7 drop-outs; last-observation-carried forward analysis performed | Low risk | None known |
| Sezer et al., 2014 [19] | Unclear (not stated) | Unclear (not stated) | High Risk (open label) | Unclear (not stated) | Unclear (not stated) | Low risk | None known |
| Goicoechea et al., 2015 * [20] Goicoechea et al., 2010 [21] | Low risk (randomization performed using a computer-generated list) | Unclear (not stated) | High Risk (single blind) | High Risk | Low risk (13 drop-outs; ITT analysis performed) | Low risk | None known |
| Bayram et al., 2015 [22] | High risk (“patients were randomized in a consecutive manner”) | Unclear (not stated) | High Risk (open label) | Unclear (not stated) | Unclear (not stated) | Low risk | None known |
| Ivanov and Ivanova, 2015 [23] | Unclear (not stated) | Unclear (not stated) | High Risk (open label) | Unclear (not stated) | Unclear (not stated) | Low risk | None known |
| Sircar et al., 2015 [24] | Low risk (randomization performed using a computer-generated random-number table) | Low risk (“allocation concealment was done by sealed sequentially numbered opaque envelopes”) | Low Risk (double blind) | Low risk (treatment assigned was not known by the investigator) | Low risk (10 drop-outs; per-protocol analysis performed) | Low risk | Low risk of funding bias (“drugs and placebo were provided by Intas Pharmaceuticals, which had no other role in funding, study design, data collection and analysis, decision to publish or preparation of the manuscript”) |
| Tanaka et al., 2015 [25] | High risk | High Risk (“simple randomization was used by drawing a sealed envelope containing the intervention allocation from a box”) | High Risk (open label) | High Risk (open label) | Low risk (5 drop-outs; per-protocol analysis performed) | Low risk | None known |
| Beddhu et al., 2016 [26] | Low risk (“randomization performed by blocks of 4 using a random number generator”) | Unclear (not stated) | Low Risk (double blind) | Low risk (“investigators and study staff were blinded to the treatment assignment”) | Low risk (4 drop-outs; ITT analysis performed) | Low risk | Low risk of funding bias (“the study was funded by a grant from Takeda Pharmaceuticals USA, Inc. The sponsor had no role in the design and conduct of the study or analysis and interpretation of results or preparation of the manuscript”) |
| Saag et al., 2016 [27] | Unclear (not stated) | Low risk (“Febuxostat and placebo tablets were overencapsulated in a similar manner to ensure blinding of study medication”) | Low Risk (double blind) | Unclear (not stated) | Low risk (efficacy and safety analyses performed by last observation carried forward method) | Low risk | High risk of funding bias (“the study was funded by Takeda Pharmaceuticals, Deerfield, IL. The sponsor authors were involved in the design and conduct of the study, all study analyses, the drafting and editing of the manuscript”) |
| Xanthine Oxidase Inhibitors versus Placebo or Standard Therapy | |||
|---|---|---|---|
| Patient or population: people with chronic kidney disease Intervention: Allopurinol, Febuxostat or Topiroxostat Comparison: placebo or standard therapy | |||
| Outcome | Effect Estimate (95% CI) | N. of Participants (Studies) | Quality of the Evidence (GRADE) |
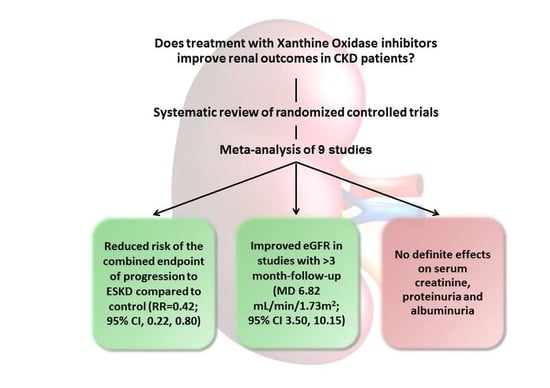
| ESKD | RR 0.42 (0.22,0.80) | 204 (3 studies) | ⊕⊕⊕⊕ High |
| Serum Creatinine | MD −0.05 (−0.12,0.02) | 270 (3 studies) | ⊕⚪⚪⚪1 Very Low |
| eGFR (all studies) (F.U. > 3 mo.) (blind design) |
|
| ⊕⚪⚪⚪1 Very Low ⊕⊕⊕⚪2 Moderate ⊕⊕⚪⚪3 Low |
| Proteinuria | SMD −0.06 (−0.39,0.26) | 191 (4 studies) | ⊕⊕⊕⊕ High |
| Albuminuria * | N/A | 303 (4 studies) | N/A |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisano, A.; Cernaro, V.; Gembillo, G.; D’Arrigo, G.; Buemi, M.; Bolignano, D. Xanthine Oxidase Inhibitors for Improving Renal Function in Chronic Kidney Disease Patients: An Updated Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2017, 18, 2283. https://doi.org/10.3390/ijms18112283
Pisano A, Cernaro V, Gembillo G, D’Arrigo G, Buemi M, Bolignano D. Xanthine Oxidase Inhibitors for Improving Renal Function in Chronic Kidney Disease Patients: An Updated Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2017; 18(11):2283. https://doi.org/10.3390/ijms18112283
Chicago/Turabian StylePisano, Anna, Valeria Cernaro, Guido Gembillo, Graziella D’Arrigo, Michele Buemi, and Davide Bolignano. 2017. "Xanthine Oxidase Inhibitors for Improving Renal Function in Chronic Kidney Disease Patients: An Updated Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 18, no. 11: 2283. https://doi.org/10.3390/ijms18112283
APA StylePisano, A., Cernaro, V., Gembillo, G., D’Arrigo, G., Buemi, M., & Bolignano, D. (2017). Xanthine Oxidase Inhibitors for Improving Renal Function in Chronic Kidney Disease Patients: An Updated Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 18(11), 2283. https://doi.org/10.3390/ijms18112283