Glucocorticoids Cause Gender-Dependent Reversal of Hepatic Fibrosis in the MDR2-Knockout Mouse Model
Abstract
:1. Introduction
2. Results
2.1. The HPA Axis Is Suppressed in MDR2KO Mice in Both Males and Females
2.2. Liver GR Was Equally Expressed in Male and Female MDR2KO and FVBN Mice
2.3. Serum Chemistry of Male and Female MDR2KO Mice as Compared to FVBN Controls
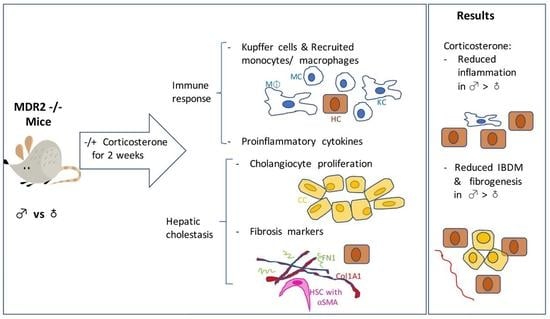
2.4. The Increased Intrahepatic Bile Duct Mass (IBDM) of MDR2KO Mice Was Reversed by Corticosterone Treatment
2.5. Corticosterone Treatment Reduced the Excess Inflammatory Hepatic Cells in MDR2KO Mice
2.6. Corticosterone Treatment Reduces Proinflammatory Cytokines in MDR2KO Mice
2.7. Corticosterone Reverses Liver Fibrosis in MDR2KO Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Treatment
4.3. Assay of HPA Axis Activity
4.4. Assessment of Cholangiocyte Proliferation, Liver Fibrosis and Inflammation
4.5. Assays of mRNA Expression of Genes with Role in Inflammation and Fibrosis by RT-qPCR
4.6. Statistical Analysis of Results
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ANIT | α-naphtylisothiocyanate |
| αSMA | Alpha smooth muscle actin |
| BA | Bile acid |
| BDL | Bile duct ligation |
| CCL2 | C chemokine ligand 2 |
| Ccr2 | C chemokine receptor 2 (receptor of CCL2) |
| Clec4f | C-lectin 4f |
| CK19 | Cytokeratin 19 |
| Col1A1 | Collagen type 1 alpha 1 |
| CRH | Corticotrope releasing hormone |
| EIA | Enzyme immune assay |
| ER-α, ER-β | Estrogen receptor-α, -β |
| FN1 | Fibronectin 1 |
| GR | Glucocorticoid receptor |
| HPA | Hypothalamus-Pituitary-Adrenal axis |
| HSC | Hepatic stellate cells |
| HSP | Heat shock protein |
| IBDM | Intrahepatic biliary duct mass |
| IHC | Immunohistochemistry |
| IL-6 | Interleukin 6 |
| MDR2KO | Multidrug resistance protein 2 knockout transgenic mouse |
| MNC | Mononuclear cells |
| PCNA | Proliferating cell nuclear antigen |
| PSC | Primary sclerosing cholangitis |
| PBC | Primary biliary cirrhosis |
| TIMP1 | Tissue inhibitor of metallopeptidases 1 |
| TNFα | Tumor necrosis factor alpha |
| UDCA | Ursodeoxycholic acid |
References
- Jacobson, L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol. Metab. Clin. N. Am. 2005, 34, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.E. HPA and immune axes in stress: Involvement of the serotonergic system. Neuroimmunomodulation 2006, 13, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Koslo, R.J.; Gmerek, D.E.; Cowan, A.; Porreca, F. Intrathecal bombesin-induced inhibition of gastrointestinal transit: Requirement for an intact pituitary-adrenal axis. Regul. Pept. 1986, 14, 237–242. [Google Scholar] [CrossRef]
- Trifan, A.; Chiriac, S.; Stanciu, C. Update on adrenal insufficiency in patients with liver cirrhosis. World J. Gastroenterol. 2013, 19, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Peng, Y.S.; Chen, Y.C.; Liu, N.J.; Ho, Y.P.; Fang, J.T.; Lien, J.M.; Yang, C.; Chen, P.C.; Wu, C.S. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology 2006, 43, 673–681. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, A.D.; Macfarlane, D.P.; O’Flaherty, E.; Livingstone, D.E.; Mitic, T.; McConnell, K.M.; McKenzie, S.M.; Davies, E.; Reynolds, R.M.; Thiesson, H.C.; et al. Bile acids modulate glucocorticoid metabolism and the hypothalamic-pituitary-adrenal axis in obstructive jaundice. J. Hepatol. 2010, 52, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.G.; Maric, M. Defective corticotropin-releasing hormone mediated neuroendocrine and behavioral responses in cholestatic rats: Implications for cholestatic liver disease-related sickness behaviors. Hepatology 1995, 22, 1560–1564. [Google Scholar] [PubMed]
- Swain, M.G.; Patchev, V.; Vergalla, J.; Chrousos, G.; Jones, E.A. Suppression of hypothalamic-pituitary-adrenal axis responsiveness to stress in a rat model of acute cholestasis. J. Clin. Investig. 1993, 91, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; Ueno, Y.; Pae, H.Y.; Huang, L.; Frampton, G.; Galindo, C.; Francis, H.; Horvat, D.; McMillin, M.; Demorrow, S. Suppression of the HPA axis during extrahepatic biliary obstruction induces cholangiocyte proliferation in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G182–G193. [Google Scholar] [CrossRef] [PubMed]
- McMillin, M.; Frampton, G.; Quinn, M.; Divan, A.; Grant, S.; Patel, N.; Newell-Rogers, K.; DeMorrow, S. Suppression of the HPA Axis During Cholestasis Can Be Attributed to Hypothalamic Bile Acid Signaling. Mol. Endocrinol. 2015, 29, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Vegiopoulos, A.; Herzig, S. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell. Endocrinol. 2007, 275, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.A.; Cidlowski, J.A. Endogenous hepatic glucocorticoid receptor signaling coordinates sex-biased inflammatory gene expression. FASEB J. 2016, 30, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Duma, D.; Collins, J.B.; Chou, J.W.; Cidlowski, J.A. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci. Signal. 2010, 3, ra74. [Google Scholar] [CrossRef] [PubMed]
- Elakovic, I.; Djordjevic, A.; Adzic, M.; Djordjevic, J.; Radojcic, M.; Matic, G. Gender-specific response of brain corticosteroid receptors to stress and fluoxetine. Brain Res. 2011, 1384, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Elakovic, I.; Vasiljevic, D.; Adzic, M.; Djordjevic, A.; Djordjevic, J.; Radojcic, M.; Matic, G. Sexually dimorphic functional alterations of rat hepatic glucocorticoid receptor in response to fluoxetine. Eur. J. Pharmacol. 2010, 632, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Mauad, T.H.; van Nieuwkerk, C.M.; Dingemans, K.P.; Smit, J.J.; Schinkel, A.H.; Notenboom, R.G.; van den Bergh Weerman, M.A.; Verkruisen, R.P.; Groen, A.K.; Oude Elferink, R.P.; et al. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am. J. Pathol. 1994, 145, 1237–1245. [Google Scholar] [PubMed]
- Trauner, M.; Fickert, P.; Wagner, M. MDR3 (ABCB4) defects: A paradigm for the genetics of adult cholestatic syndromes. Semin. Liver Dis. 2007, 27, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Katzenellenbogen, M.; Mizrahi, L.; Pappo, O.; Klopstock, N.; Olam, D.; Jacob-Hirsch, J.; Amariglio, N.; Rechavi, G.; Domany, E.; Galun, E.; et al. Molecular mechanisms of liver carcinogenesis in the mdr2-knockout mice. Mol. Cancer Res. 2007, 5, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Hines, J.E.; Johnson, S.J.; Burt, A.D. In vivo responses of macrophages and perisinusoidal cells to cholestatic liver injury. Am. J. Pathol. 1993, 142, 511–518. [Google Scholar] [PubMed]
- Kinoshita, M.; Uchida, T.; Sato, A.; Nakashima, M.; Nakashima, H.; Shono, S.; Habu, Y.; Miyazaki, H.; Hiroi, S.; Seki, S. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J. Hepatol. 2010, 53, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Roland, C.R.; Goss, J.A.; Mangino, M.J.; Hafenrichter, D.; Flye, M.W. Autoregulation by eicosanoids of human Kupffer cell secretory products: A study of interleukin-1, interleukin-6, tumor necrosis factor-alpha, transforming growth factor-beta, and nitric oxide. Ann. Surg. 1994, 219, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N. Engl. J. Med. 1993, 328, 1828–1835. [Google Scholar] [PubMed]
- Ramm, G.A.; Nair, V.G.; Bridle, K.R.; Shepherd, R.W.; Crawford, D.H. Contribution of hepatic parenchymal and nonparenchymal cells to hepatic fibrogenesis in biliary atresia. Am. J. Pathol. 1998, 153, 527–535. [Google Scholar] [CrossRef]
- Smit, J.J.; Schinkel, A.H.; Oude Elferink, R.P.; Groen, A.K.; Wagenaar, E.; van Deemter, L.; Mol, C.A.; Ottenhoff, R.; van der Lugt, N.M.; van Roon, M.A.; et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 1993, 75, 451–462. [Google Scholar] [CrossRef]
- Lammert, F.; Wang, D.Q.; Hillebrandt, S.; Geier, A.; Fickert, P.; Trauner, M.; Matern, S.; Paigen, B.; Carey, M.C. Spontaneous cholecysto- and hepatolithiasis in Mdr2-/- mice: A model for low phospholipid-associated cholelithiasis. Hepatology 2004, 39, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Learned, R.M.; Rossi, S.J.; DePaoli, A.M.; Tian, H.; Ling, L. Engineered fibroblast growth factor 19 reduces liver injury and resolves sclerosing cholangitis in Mdr2-deficient mice. Hepatology 2016, 63, 914–929. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, E.; Cresteil, D.; Manouvrier, S.; Boute, O.; Hadchouel, M. Heterozygous non-sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet 1999, 353, 210–211. [Google Scholar] [CrossRef]
- Rosmorduc, O.; Hermelin, B.; Poupon, R. MDR3 gene defect in adults with symptomatic intrahepatic and gallbladder cholesterol cholelithiasis. Gastroenterology 2001, 120, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, E.; De Vree, J.M.; Cresteil, D.; Sokal, E.M.; Sturm, E.; Dumont, M.; Scheffer, G.L.; Paul, M.; Burdelski, M.; Bosma, P.J.; et al. The wide spectrum of multidrug resistance 3 deficiency: From neonatal cholestasis to cirrhosis of adulthood. Gastroenterology 2001, 120, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, R.; Yang, J.; Sun, L.; Zhang, L.; Jiang, Z.; Puri, P.; Gurley, E.C.; Lai, G.; Tang, Y.; et al. The role of long noncoding RNA H19 in gender disparity of cholestatic liver injury in multidrug resistance 2 gene knockout mice. Hepatology 2017, 66, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Smyk, D.S.; Rigopoulou, E.I.; Pares, A.; Billinis, C.; Burroughs, A.K.; Muratori, L.; Invernizzi, P.; Bogdanos, D.P. Sex differences associated with primary biliary cirrhosis. Clin. Dev. Immunol. 2012, 2012, 610504. [Google Scholar] [CrossRef] [PubMed]
- Smyk, D.S.; Rigopoulou, E.I.; Lleo, A.; Abeles, R.D.; Mavropoulos, A.; Billinis, C.; Invernizzi, P.; Bogdanos, D.P. Immunopathogenesis of primary biliary cirrhosis: An old wives’ tale. Immun. Ageing 2011, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, D.; Invernizzi, P.; Onori, P.; Franchitto, A.; De Santis, A.; Crosignani, A.; Sferra, R.; Ginanni-Corradini, S.; Mancino, M.G.; Maggioni, M.; et al. Estrogen receptors in cholangiocytes and the progression of primary biliary cirrhosis. J. Hepatol. 2004, 41, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, L.; He, M.M.; Liu, Z.F.; Gao, B.X.; Wang, X.D. Corticotropin-releasing hormone expression in patients with intrahepatic cholestasis of pregnancy after ursodeoxycholic acid treatment: An initial experience. Curr. Med. Res. Opin. 2014, 30, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Boberg, K.M.; Egeland, T.; Schrumpf, E. Long-term effect of corticosteroid treatment in primary sclerosing cholangitis patients. Scand. J. Gastroenterol. 2003, 38, 991–995. [Google Scholar] [PubMed]
- Van Hoogstraten, H.J.; Vleggaar, F.P.; Boland, G.J.; van Steenbergen, W.; Griffioen, P.; Hop, W.C.; van Hattum, J.; van Berge Henegouwen, G.P.; Schalm, S.W.; van Buuren, H.R. Budesonide or prednisone in combination with ursodeoxycholic acid in primary sclerosing cholangitis: A randomized double-blind pilot study. Belgian-Dutch PSC Study Group. Am. J. Gastroenterol. 2000, 95, 2015–2022. [Google Scholar] [CrossRef]
- Bourke, C.H.; Raees, M.Q.; Malviya, S.; Bradburn, C.A.; Binder, E.B.; Neigh, G.N. Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology 2013, 38, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Bourke, C.H.; Harrell, C.S.; Neigh, G.N. Stress-induced sex differences: Adaptations mediated by the glucocorticoid receptor. Horm. Behav. 2012, 62, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Tracy, T.F., Jr.; Dillon, P.; Fox, E.S.; Minnick, K.; Vogler, C. The inflammatory response in pediatric biliary disease: Macrophage phenotype and distribution. J. Pediatr. Surg. 1996, 31, 121–125. [Google Scholar] [CrossRef]
- Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017, 66, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, G.W.; Hill, R.L. Structure of the gene for a carbohydrate-binding receptor unique to rat kupffer cells. J. Biol. Chem. 1991, 266, 1850–1857. [Google Scholar] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Tanner, A.R.; Powell, L.W. Corticosteroids in liver disease: Possible mechanisms of action, pharmacology, and rational use. Gut 1979, 20, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Dourakis, S.P.; Mayroyannis, C.; Alexopoulou, A.; Hadziyannis, S.J. Prolonged cholestatic jaundice after endoscopic retrograde cholangiography. Hepatogastroenterology 1997, 44, 677–680. [Google Scholar] [PubMed]
- Dunn, J.M.; McNair, A. Prolonged cholestasis following successful removal of common bile duct stones: Beware patients on estrogen therapy. World J. Gastroenterol. 2007, 13, 6277–6280. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, D.J.; Wallace, R.B.; Rodabough, R.J.; Greenland, P.; LaCroix, A.Z.; Limacher, M.C.; Larson, J.C. Effect of estrogen therapy on gallbladder disease. JAMA 2005, 293, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.M. Physical and metabolic factors in gallstone pathogenesis. Gastroenterol. Clin. N. Am. 1999, 28, 75–97. [Google Scholar] [CrossRef]
- Verhulst, S.; Best, J.; Syn, W.K.; Reynaert, H.; Hellemans, K.H.; Canbay, A.; Dolle, L.; van Grunsven, L.A. Infliximab and Dexamethasone Attenuate the Ductular Reaction in Mice. Sci. Rep. 2016, 6, 36586. [Google Scholar] [CrossRef] [PubMed]
- Tiao, M.M.; Lin, T.K.; Chen, J.B.; Liou, C.W.; Wang, P.W.; Huang, C.C.; Chou, Y.M.; Huang, Y.H.; Chuang, J.H. Dexamethasone decreases cholestatic liver injury via inhibition of intrinsic pathway with simultaneous enhancement of mitochondrial biogenesis. Steroids 2011, 76, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Tuckermann, J.P.; Kleiman, A.; McPherson, K.G.; Reichardt, H.M. Molecular mechanisms of glucocorticoids in the control of inflammation and lymphocyte apoptosis. Crit. Rev. Clin. Lab. Sci. 2005, 42, 71–104. [Google Scholar] [CrossRef] [PubMed]
- Tuckermann, J.P.; Kleiman, A.; Moriggl, R.; Spanbroek, R.; Neumann, A.; Illing, A.; Clausen, B.E.; Stride, B.; Forster, I.; Habenicht, A.J.; et al. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J. Clin. Investig. 2007, 117, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Klassen, C.; Karabinskaya, A.; Dejager, L.; Vettorazzi, S.; Van Moorleghem, J.; Luhder, F.; Meijsing, S.H.; Tuckermann, J.P.; Bohnenberger, H.; Libert, C.; et al. Airway Epithelial Cells Are Crucial Targets of Glucocorticoids in a Mouse Model of Allergic Asthma. J. Immunol. 2017, 199, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, H.E.; Wang, Y.; Princen, H.; Romijn, J.A.; Havekes, L.M.; Smit, J.W.; Meijer, O.C.; Biermasz, N.R.; Rensen, P.C.; Pereira, A.M. Both transient and continuous corticosterone excess inhibit atherosclerotic plaque formation in APOE*3-leiden.CETP mice. PLoS ONE 2013, 8, e63882. [Google Scholar] [CrossRef] [PubMed] [Green Version]











© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrescu, A.D.; Grant, S.; Frampton, G.; Kain, J.; Hadidi, K.; Williams, E.; McMillin, M.; DeMorrow, S. Glucocorticoids Cause Gender-Dependent Reversal of Hepatic Fibrosis in the MDR2-Knockout Mouse Model. Int. J. Mol. Sci. 2017, 18, 2389. https://doi.org/10.3390/ijms18112389
Petrescu AD, Grant S, Frampton G, Kain J, Hadidi K, Williams E, McMillin M, DeMorrow S. Glucocorticoids Cause Gender-Dependent Reversal of Hepatic Fibrosis in the MDR2-Knockout Mouse Model. International Journal of Molecular Sciences. 2017; 18(11):2389. https://doi.org/10.3390/ijms18112389
Chicago/Turabian StylePetrescu, Anca D., Stephanie Grant, Gabriel Frampton, Jessica Kain, Karam Hadidi, Elaina Williams, Matthew McMillin, and Sharon DeMorrow. 2017. "Glucocorticoids Cause Gender-Dependent Reversal of Hepatic Fibrosis in the MDR2-Knockout Mouse Model" International Journal of Molecular Sciences 18, no. 11: 2389. https://doi.org/10.3390/ijms18112389
APA StylePetrescu, A. D., Grant, S., Frampton, G., Kain, J., Hadidi, K., Williams, E., McMillin, M., & DeMorrow, S. (2017). Glucocorticoids Cause Gender-Dependent Reversal of Hepatic Fibrosis in the MDR2-Knockout Mouse Model. International Journal of Molecular Sciences, 18(11), 2389. https://doi.org/10.3390/ijms18112389