Dynamic Reorganization of the Cytoskeleton during Apoptosis: The Two Coffins Hypothesis
Abstract
:1. Introduction: An Overview of Apoptosis
2. Genotoxic Cell Response and Cytoskeleton
3. Cytoskeleton Rearrangements during the Execution Phase of Apoptosis
3.1. Reorganization of Microtubules during Apoptosis
3.2. Reorganization of Actin Cytoskeleton during Apoptosis
3.3. Reorganization of Intermediate Filaments during Apoptosis
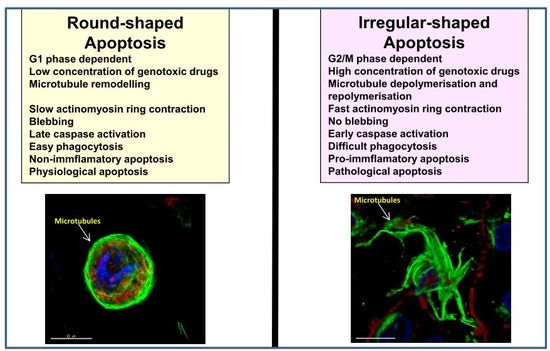
4. Modulation of Round and Irregular-Shaped Apoptosis
5. Biological Implications of the “Two Coffins” Model in Apoptosis
6. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Meier, P.; Finch, A.; Evan, G. Apoptosis in development. Nature 2000, 407, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2014, 147, 742–758. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.D.; Weil, M.; Raff, M.C. Programmed cell death in animal development. Cell 1997, 88, 347–354. [Google Scholar] [CrossRef]
- Narayanan, V. Apoptosis in development and disease of the nervous system: 1. Naturally occurring cell death in the developing nervous system. Pediatr. Neurol. 1997, 16, 9–13. [Google Scholar] [CrossRef]
- Burg, M.L.; Chai, Y.; Yao, C.A.; Magee, W., 3rd; Figueiredo, J.C. Epidemiology, etiology, and treatment of isolated cleft palate. Front. Physiol. 2016, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Ellis, H.M.; Horvitz, H.R. Genetic control of programmed cell death in the nematode C. elegans. Cell 1986, 44, 817–829. [Google Scholar] [CrossRef]
- Xu, D.; Woodfield, S.E.; Lee, T.V.; Fan, Y.; Antonio, C.; Bergmann, A. Genetic control of programmed cell death (apoptosis) in drosophila. Fly 2009, 3, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Thompson, C.B. Apoptosis and disease: Regulation and clinical relevance of programmed cell death. Annu. Rev. Med. 1997, 48, 267–281. [Google Scholar] [PubMed]
- Silva, M.T. Secondary necrosis: The natural outcome of the complete apoptotic program. FEBS Lett. 2010, 584, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.T.; do Vale, A.; dos Santos, N.M. Secondary necrosis in multicellular animals: An outcome of apoptosis with pathogenic implications. Apoptosis 2008, 13, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Logue, S.E.; Martin, S.J. Caspase activation cascades in apoptosis. Biochem. Soc. Trans. 2008, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R. Apoptosis: Unmasking the executioner. Cell Death Differ. 1998, 5, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.K.; Hulett, M.D.; Parish, C.R. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ. 2010, 17, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; D'Herde, K.; Vandenabeele, P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis 2006, 11, 1709–1726. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Calvi, B.R. Different cell cycle modifications repress apoptosis at different steps independent of developmental signaling in drosophila. Mol. Biol. Cell 2016, 27, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. CR 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Helleday, T.; Petermann, E.; Lundin, C.; Hodgson, B.; Sharma, R.A. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 2008, 8, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.; Turchi, J.J. Chemotherapy induced DNA damage response: Convergence of drugs and pathways. Cancer Biol. Ther. 2013, 14, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Marechal, A.; Zou, L. DNA damage sensing by the atm and atr kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef] [PubMed]
- Furgason, J.M.; Bahassi el, M. Targeting DNA repair mechanisms in cancer. Pharmacol. Ther. 2013, 137, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald, E.R., 3rd; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. Atm and atr substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Matt, S.; Hofmann, T.G. The DNA damage-induced cell death response: A roadmap to kill cancer cells. Cell Mol. Life Sci. 2016, 73, 2829–2850. [Google Scholar] [CrossRef] [PubMed]
- Follis, A.V.; Llambi, F.; Merritt, P.; Chipuk, J.E.; Green, D.R.; Kriwacki, R.W. Pin1-induced proline isomerization in cytosolic p53 mediates bax activation and apoptosis. Mol. Cell 2015, 59, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M. Rho signalling at a glance. J. Cell Sci. 2004, 117, 5457–5458. [Google Scholar] [CrossRef] [PubMed]
- Iden, S.; Collard, J.G. Crosstalk between small gtpases and polarity proteins in cell polarization. Nat. Rev. Mol. Cell Biol. 2008, 9, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Kjoller, L.; Hall, A. Signaling to rho gtpases. Exp. Cell Res. 1999, 253, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.M.; Takai, S.; Yamada, K.; Miki, T. Isolation of a novel oncogene, net1, from neuroepithelioma cells by expression cDNA cloning. Oncogene 1996, 12, 1259–1266. [Google Scholar] [PubMed]
- Oh, W.; Frost, J.A. Rho gtpase independent regulation of atm activation and cell survival by the rhogef net1a. Cell Cycle 2014, 13, 2765–2772. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Carr, H.S.; Richter-Dahlfors, A.; Masucci, M.G.; Thelestam, M.; Frost, J.A.; Frisan, T. A bacterial cytotoxin identifies the RhoA exchange factor Net1 as a key effector in the response to DNA damage. PLoS ONE 2008, 3, e2254. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-kinase/rock: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Perruche, S.; Saae, P. L14. Immunomodulatory properties of apoptotic cells. Presse Med. 2013, 42, 537–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, J.C.; Stone, N.L.; Pittman, R.N. Extranuclear apoptosis. The role of the cytoplasm in the execution phase. J. Cell Biol. 1999, 146, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Ndozangue-Touriguine, O.; Hamelin, J.; Breard, J. Cytoskeleton and apoptosis. Biochem. Pharmacol. 2008, 76, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.K.; Lane, J.D. Microtubules: Forgotten players in the apoptotic execution phase. Trends Cell Biol. 2006, 16, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Pittman, S.; Geyp, M.; Fraser, M.; Ellem, K.; Peaston, A.; Ireland, C. Multiple centrosomal microtubule organising centres and increased microtubule stability are early features of vp-16-induced apoptosis in ccrf-cem cells. Leuk. Res. 1997, 21, 491–499. [Google Scholar] [CrossRef]
- Pittman, S.M.; Strickland, D.; Ireland, C.M. Polymerization of tubulin in apoptotic cells is not cell cycle dependent. Exp. Cell Res. 1994, 215, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Desouza, M.; Gunning, P.W.; Stehn, J.R. The actin cytoskeleton as a sensor and mediator of apoptosis. Bioarchitecture 2012, 2, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Van Engeland, M.; Kuijpers, H.J.; Ramaekers, F.C.; Reutelingsperger, C.P.; Schutte, B. Plasma membrane alterations and cytoskeletal changes in apoptosis. Exp. Cell Res. 1997, 235, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.K.; Betin, V.M.; Malesinski, S.D.; Lane, J.D. A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J. Cell Sci. 2006, 119, 2362–2374. [Google Scholar] [CrossRef] [PubMed]
- Oropesa-Avila, M.; de la Cruz-Ojeda, P.; Porcuna, J.; Villanueva-Paz, M.; Fernandez-Vega, A.; de la Mata, M.; de Lavera, I.; Rivero, J.M.; Luzon-Hidalgo, R.; Alvarez-Cordoba, M.; et al. Two coffins and a funeral: Early or late caspase activation determines two types of apoptosis induced by DNA damaging agents. Apoptosis 2017, 22, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Brady, H.J.; Gil-Gomez, G.; Kirberg, J.; Berns, A.J. Bax α perturbs t cell development and affects cell cycle entry of t cells. EMBO J. 1996, 15, 6991–7001. [Google Scholar] [PubMed]
- North, S.; Hainaut, P. P53 and cell-cycle control: A finger in every pie. Pathol. Biol. 2000, 48, 255–270. [Google Scholar] [PubMed]
- Vairo, G.; Soos, T.J.; Upton, T.M.; Zalvide, J.; DeCaprio, J.A.; Ewen, M.E.; Koff, A.; Adams, J.M. Bcl-2 retards cell cycle entry through p27(kip1), prb relative p130, and altered e2f regulation. Mol. Cell. Biol. 2000, 20, 4745–4753. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Cell death beyond apoptosis. Leukemia 2000, 14, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.; Willgohs, E.; Nguyen, T.N.; Khan, E.M.; Goldkorn, T. Monte carlo simulation of cell death signaling predicts large cell-to-cell stochastic fluctuations through the type 2 pathway of apoptosis. Biophys. J. 2008, 95, 3559–3562. [Google Scholar] [CrossRef] [PubMed]
- Broker, L.E.; Huisman, C.; Ferreira, C.G.; Rodriguez, J.A.; Kruyt, F.A.; Giaccone, G. Late activation of apoptotic pathways plays a negligible role in mediating the cytotoxic effects of discodermolide and epothilone b in non-small cell lung cancer cells. Cancer Res. 2002, 62, 4081–4088. [Google Scholar] [PubMed]
- Etienne-Manneville, S. From signaling pathways to microtubule dynamics: The key players. Curr. Opin. Cell Biol. 2010, 22, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Xie, M.; Shah, V.R.; Schneider, M.D.; Entman, M.L.; Wei, L.; Schwartz, R.J. Activation of Rho-associated coiled-coil protein kinase 1 (rock-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc. Natl. Acad. Sci. USA 2006, 103, 14495–14500. [Google Scholar] [CrossRef] [PubMed]
- Bonfoco, E.; Zhivotovsky, B.; Orrenius, S.; Lipton, A.; Nicotera, P. Cytoskeletal breakdown and apoptosis elicited by no donors in cerebellar granule cells require nmda receptor activation. J Neurochem. 1996, 67, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Golsteyn, R.M. Cdk1 and Cdk2 complexes (cyclin dependent kinases) in apoptosis: A role beyond the cell cycle. Cancer Lett. 2005, 217, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alcazar, J.A.; Rodriguez-Hernandez, A.; Cordero, M.D.; Fernandez-Ayala, D.J.; Brea-Calvo, G.; Garcia, K.; Navas, P. The apoptotic microtubule network preserves plasma membrane integrity during the execution phase of apoptosis. Apoptosis 2007, 12, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Zuo, D.; Jiang, X.; Han, M.; Shen, J.; Lang, B.; Guan, Q.; Bai, Z.; Han, C.; Li, Z.; Zhang, W.; et al. Methyl 5-[(1h-indol-3-yl)selanyl]-1h-benzoimidazol-2-ylcarbamate (m-24), a novel tubulin inhibitor, causes g2/m arrest and cell apoptosis by disrupting tubulin polymerization in human cervical and breast cancer cells. Toxicol. In Vitro 2017, 42, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Janicke, R.U.; Schulze-Osthoff, K. Many cuts to ruin: A comprehensive update of caspase substrates. Cell Death Differ. 2003, 10, 76–100. [Google Scholar] [CrossRef] [PubMed]
- Gerner, C.; Frohwein, U.; Gotzmann, J.; Bayer, E.; Gelbmann, D.; Bursch, W.; Schulte-Hermann, R. The fas-induced apoptosis analyzed by high throughput proteome analysis. J. Biol. Chem. 2000, 275, 39018–39026. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Dictenberg, J.B.; Purohit, A.; Tuft, R.; Doxsey, S.J. Cytoplasmic dynein-mediated assembly of pericentrin and γ tubulin onto centrosomes. Mol. Biol. Cell 2000, 11, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.S. Spindle assembly and the art of regulating microtubule dynamics by maps and stathmin/op18. Trends Cell Biol. 2000, 10, 261–267. [Google Scholar] [CrossRef]
- Carazo-Salas, R.E.; Gruss, O.J.; Mattaj, I.W.; Karsenti, E. Ran-gtp coordinates regulation of microtubule nucleation and dynamics during mitotic-spindle assembly. Nat. Cell Biol. 2001, 3, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Galjart, N.; Perez, F. A plus-end raft to control microtubule dynamics and function. Curr. Opin. Cell Biol. 2003, 15, 48–53. [Google Scholar] [CrossRef]
- Lamb, N.J.; Fernandez, A.; Watrin, A.; Labbe, J.C.; Cavadore, J.C. Microinjection of p34cdc2 kinase induces marked changes in cell shape, cytoskeletal organization, and chromatin structure in mammalian fibroblasts. Cell 1990, 60, 151–165. [Google Scholar] [CrossRef]
- Sugawara, E.; Nikaido, H. Properties of adeabc and adeijk efflux systems of acinetobacter baumannii compared with those of the acrab-tolc system of Escherichia coli. Antimicrob. Agents Chemother. 2014, 58, 7250–7257. [Google Scholar] [CrossRef] [PubMed]
- Fourest-Lieuvin, A.; Peris, L.; Gache, V.; Garcia-Saez, I.; Juillan-Binard, C.; Lantez, V.; Job, D. Microtubule regulation in mitosis: Tubulin phosphorylation by the cyclin-dependent kinase Cdk1. Mol. Biol. Cell 2006, 17, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.C.; Lee, V.M.; Pittman, R.N. Activation of a PP2A-like phosphatase and dephosphorylation of tau protein characterize onset of the execution phase of apoptosis. J. Cell Sci. 1998, 111, 625–636. [Google Scholar] [PubMed]
- Jiang, Y. Regulation of the cell cycle by protein phosphatase 2a in saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.D.; Lucocq, J.; Pryde, J.; Barr, F.A.; Woodman, P.G.; Allan, V.J.; Lowe, M. Caspase-mediated cleavage of the stacking protein grasp65 is required for golgi fragmentation during apoptosis. J. Cell Biol. 2002, 156, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Gromley, A.; Jurczyk, A.; Sillibourne, J.; Halilovic, E.; Mogensen, M.; Groisman, I.; Blomberg, M.; Doxsey, S. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into s phase. J. Cell Biol. 2003, 161, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Wilde, A.; Lizarraga, S.B.; Zhang, L.; Wiese, C.; Gliksman, N.R.; Walczak, C.E.; Zheng, Y. Ran stimulates spindle assembly by altering microtubule dynamics and the balance of motor activities. Nat. Cell Biol. 2001, 3, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992, 70, 389–399. [Google Scholar] [CrossRef]
- Coleman, M.L.; Olson, M.F. Rho gtpase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ. 2002, 9, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, T.; Wilm, M.; Karsenti, E.; Vernos, I. Tpx2, a novel xenopus map involved in spindle pole organization. J. Cell Biol. 2000, 149, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Wickman, G.; Julian, L.; Olson, M.F. How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ. 2012, 19, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Wickman, G.R.; Julian, L.; Mardilovich, K.; Schumacher, S.; Munro, J.; Rath, N.; Zander, S.A.; Mleczak, A.; Sumpton, D.; Morrice, N.; et al. Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ. 2013, 20, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Gourlay, C.W.; Ayscough, K.R. The actin cytoskeleton in ageing and apoptosis. FEMS Yeast Res. 2005, 5, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Hall, A. The rho exchange factor net1 is regulated by nuclear sequestration. J. Biol. Chem. 2002, 277, 14581–14588. [Google Scholar] [CrossRef] [PubMed]
- Dubash, A.D.; Guilluy, C.; Srougi, M.C.; Boulter, E.; Burridge, K.; Garcia-Mata, R. The small gtpase rhoa localizes to the nucleus and is activated by Net1 and DNA damage signals. PLoS ONE 2011, 6, e17380. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Ashok, V.; Mohanty, S.; Das, A.; Das, S.; Kumar, S.; Sen, S.; Purwar, R. Blockade of rho-associated protein kinase (rock) inhibits the contractility and invasion potential of cancer stem like cells. Oncotarget 2017, 8, 21418–21428. [Google Scholar] [CrossRef] [PubMed]
- Somlyo, A.P.; Somlyo, A.V. Signal transduction by g-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin ii. J. Physiol. 2000, 522 Pt 2, 177–185. [Google Scholar] [CrossRef]
- Byun, Y.; Chen, F.; Chang, R.; Trivedi, M.; Green, K.J.; Cryns, V.L. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ. 2001, 8, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chang, R.; Trivedi, M.; Capetanaki, Y.; Cryns, V.L. Caspase proteolysis of desmin produces a dominant-negative inhibitor of intermediate filaments and promotes apoptosis. J. Biol. Chem. 2003, 278, 6848–6853. [Google Scholar] [CrossRef] [PubMed]
- Marceau, N.; Schutte, B.; Gilbert, S.; Loranger, A.; Henfling, M.E.; Broers, J.L.; Mathew, J.; Ramaekers, F.C. Dual roles of intermediate filaments in apoptosis. Exp. Cell Res. 2007, 313, 2265–2281. [Google Scholar] [CrossRef] [PubMed]
- Erwig, L.P.; Henson, P.M. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008, 15, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Oropesa-Avila, M.; Fernandez-Vega, A.; de la Mata, M.; Maraver, J.G.; Cordero, M.D.; Cotan, D.; de Miguel, M.; Calero, C.P.; Paz, M.V.; Pavon, A.D.; et al. Apoptotic microtubules delimit an active caspase free area in the cellular cortex during the execution phase of apoptosis. Cell Death Dis. 2013, 4, e527. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Povea-Cabello, S.; Oropesa-Ávila, M.; De la Cruz-Ojeda, P.; Villanueva-Paz, M.; De la Mata, M.; Suárez-Rivero, J.M.; Álvarez-Córdoba, M.; Villalón-García, I.; Cotán, D.; Ybot-González, P.; et al. Dynamic Reorganization of the Cytoskeleton during Apoptosis: The Two Coffins Hypothesis. Int. J. Mol. Sci. 2017, 18, 2393. https://doi.org/10.3390/ijms18112393
Povea-Cabello S, Oropesa-Ávila M, De la Cruz-Ojeda P, Villanueva-Paz M, De la Mata M, Suárez-Rivero JM, Álvarez-Córdoba M, Villalón-García I, Cotán D, Ybot-González P, et al. Dynamic Reorganization of the Cytoskeleton during Apoptosis: The Two Coffins Hypothesis. International Journal of Molecular Sciences. 2017; 18(11):2393. https://doi.org/10.3390/ijms18112393
Chicago/Turabian StylePovea-Cabello, Suleva, Manuel Oropesa-Ávila, Patricia De la Cruz-Ojeda, Marina Villanueva-Paz, Mario De la Mata, Juan Miguel Suárez-Rivero, Mónica Álvarez-Córdoba, Irene Villalón-García, David Cotán, Patricia Ybot-González, and et al. 2017. "Dynamic Reorganization of the Cytoskeleton during Apoptosis: The Two Coffins Hypothesis" International Journal of Molecular Sciences 18, no. 11: 2393. https://doi.org/10.3390/ijms18112393
APA StylePovea-Cabello, S., Oropesa-Ávila, M., De la Cruz-Ojeda, P., Villanueva-Paz, M., De la Mata, M., Suárez-Rivero, J. M., Álvarez-Córdoba, M., Villalón-García, I., Cotán, D., Ybot-González, P., & Sánchez-Alcázar, J. A. (2017). Dynamic Reorganization of the Cytoskeleton during Apoptosis: The Two Coffins Hypothesis. International Journal of Molecular Sciences, 18(11), 2393. https://doi.org/10.3390/ijms18112393