Review: Receptor Targeted Nuclear Imaging of Breast Cancer
Abstract
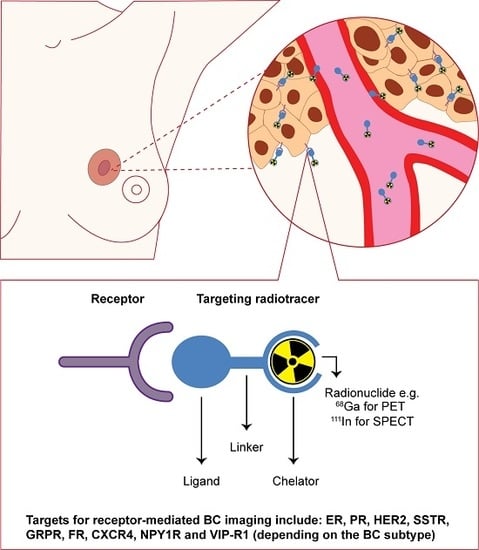
:1. Introduction
2. Targeting of Hormone Receptors for Nuclear Imaging
3. Human Epidermal Growth Factor Receptor 2 (HER2)-Targeted Imaging
4. Somatostatin Receptor (SSTR)-Mediated BC Imaging
5. Gastrin Releasing Peptide Receptor (GRPR)-Mediated BC Imaging
6. Other Targets
7. Future Outlooks
8. Conclusions
Author Contributions
Conflicts of Interest
References
- Ali, S.; Coombes, R.C. Endocrine-responsive breast cancer and strategies for combating resistance. Nat. Rev. Cancer 2002, 2, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Steeg, P.S.; Price, J.E.; Krishnamurthy, S.; Mani, S.A.; Reuben, J.; Cristofanilli, M.; Dontu, G.; Bidaut, L.; Valero, V.; et al. Breast cancer metastasis: Challenges and opportunities. Cancer Res. 2009, 69, 4951–4953. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Campana, D.; Tomassetti, P.; Fanti, S. 68Ga-labelled peptides for diagnosis of gastroenteropancreatic net. Eur. J. Nucl. Med. Mol. Imaging 2012, 39 (Suppl. S1), S52–S60. [Google Scholar] [CrossRef] [PubMed]
- Bison, S.M.; Konijnenberg, M.W.; Melis, M.; Pool, S.E.; Bernsen, M.R.; Teunissen, J.J.; Kwekkeboom, D.J.; de Jong, M. Peptide receptor radionuclide therapy using radiolabeled somatostatin analogs: Focus on future developments. Clin. Transl. Imaging 2014, 2, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.K.; Nekolla, S.; Ziegler, S.; Beer, A.; Krause, B.J.; Herrmann, K.; Scheidhauer, K.; Wester, H.J.; Rummeny, E.J.; Schwaiger, M.; et al. Spect/ct. J. Nucl. Med. 2008, 49, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Von Schulthess, G.K.; Steinert, H.C.; Hany, T.F. Integrated PET/CT: Current applications and future directions. Radiology 2006, 238, 405–422. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.A.; Turkington, T.G. Spect/CT physical principles and attenuation correction. J. Nucl. Med. Technol. 2008, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.I. Positron emission tomography: Principles, technology, and recent developments. Nucl. Phys. A 2005, 752, 679c–687c. [Google Scholar] [CrossRef]
- Kalinyak, J.E.; Berg, W.A.; Schilling, K.; Madsen, K.S.; Narayanan, D.; Tartar, M. Breast cancer detection using high-resolution breast PET compared to whole-body PET or PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.F.; Freese, D.L.; Levin, C.S. Breast-dedicated radionuclide imaging systems. J. Nucl. Med. 2016, 57 (Suppl. S1), 40S–45S. [Google Scholar] [CrossRef] [PubMed]
- Kiesewetter, D.O.; Kilbourn, M.R.; Landvatter, S.W.; Heiman, D.F.; Katzenellenbogen, J.A.; Welch, M.J. Preparation of four fluorine-18-labeled estrogens and their selective uptakes in target tissues of immature rats. J. Nucl. Med. 1984, 25, 1212–1221. [Google Scholar] [PubMed]
- Mintun, M.A.; Welch, M.J.; Siegel, B.A.; Mathias, C.J.; Brodack, J.W.; McGuire, A.H.; Katzenellenbogen, J.A. Breast cancer: PET imaging of estrogen receptors. Radiology 1988, 169, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.M.; Mankoff, D.A.; Lawton, T.; Yagle, K.; Schubert, E.K.; Stekhova, S.; Gown, A.; Link, J.M.; Tewson, T.; Krohn, K.A. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J. Nucl. Med. 2008, 49, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, J.E.; Dehdashti, F.; Siegel, B.A.; Katzenellenbogen, J.A.; Fracasso, P.; Welch, M.J. Positron emission tomography with 2-[18F]Fluoro-2-deoxy-d-glucose and 16α-[18F]fluoro-17β-estradiol in breast cancer: Correlation with estrogen receptor status and response to systemic therapy. Clin. Cancer Res. 1996, 2, 933–939. [Google Scholar] [PubMed]
- Dehdashti, F.; Mortimer, J.E.; Siegel, B.A.; Griffeth, L.K.; Bonasera, T.J.; Fusselman, M.J.; Detert, D.D.; Cutler, P.D.; Katzenellenbogen, J.A.; Welch, M.J. Positron tomographic assessment of estrogen receptors in breast cancer: Comparison with FDG-PET and in vitro receptor assays. J. Nucl. Med. 1995, 36, 1766–1774. [Google Scholar] [PubMed]
- Gemignani, M.L.; Patil, S.; Seshan, V.E.; Sampson, M.; Humm, J.L.; Lewis, J.S.; Brogi, E.; Larson, S.M.; Morrow, M.; Pandit-Taskar, N. Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer. J. Nucl. Med. 2013, 54, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, F.; Mortimer, J.E.; Trinkaus, K.; Naughton, M.J.; Ellis, M.; Katzenellenbogen, J.A.; Welch, M.J.; Siegel, B.A. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast Cancer Res. Treat. 2009, 113, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Linden, H.M.; Stekhova, S.A.; Link, J.M.; Gralow, J.R.; Livingston, R.B.; Ellis, G.K.; Petra, P.H.; Peterson, L.M.; Schubert, E.K.; Dunnwald, L.K.; et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J. Clin. Oncol. 2006, 24, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, J.E.; Dehdashti, F.; Siegel, B.A.; Trinkaus, K.; Katzenellenbogen, J.A.; Welch, M.J. Metabolic flare: Indicator of hormone responsiveness in advanced breast cancer. J. Clin. Oncol. 2001, 19, 2797–2803. [Google Scholar] [PubMed]
- Dehdashti, F.; Flanagan, F.L.; Mortimer, J.E.; Katzenellenbogen, J.A.; Welch, M.J.; Siegel, B.A. Positron emission tomographic assessment of “metabolic flare” to predict response of metastatic breast cancer to antiestrogen therapy. Eur. J. Nucl. Med. 1999, 26, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Curran, S.D.; Muellner, A.U.; Schwartz, L.H. Imaging response assessment in oncology. Cancer Imaging 2006, 6, S126–S130. [Google Scholar] [CrossRef] [PubMed]
- Lebron, L.; Greenspan, D.; Pandit-Taskar, N. PET imaging of breast cancer: Role in patient management. PET Clin. 2015, 10, 159–195. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, M.; Yang, Z.; Zhang, J.; Zhang, Y.; Luo, J.; Zhang, Y. Comparison of 18F-FES, 18F-FDG, and 18F-FMISO PET imaging probes for early prediction and monitoring of response to endocrine therapy in a mouse xenograft model of ER-positive breast cancer. PLoS ONE 2016, 11, e0159916. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.; Gano, L.; Morais, G.R.; Thiemann, T.; Oliveira, M.C. Progesterone receptor targeting with radiolabelled steroids: An approach in predicting breast cancer response to therapy. J. Steroid Biochem. Mol. Biol. 2013, 137, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.M.; Clark, A.S.; Katzenellenbogen, J.A.; Linden, H.M.; Dehdashti, F. Imaging diagnostic and therapeutic targets: Steroid receptors in breast cancer. J. Nucl. Med. 2016, 57 (Suppl. S1), 75s–80s. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, F.; Laforest, R.; Gao, F.; Aft, R.L.; Dence, C.S.; Zhou, D.; Shoghi, K.I.; Siegel, B.A.; Katzenellenbogen, J.A.; Welch, M.J. Assessment of progesterone receptors in breast carcinoma by PET with 21–18F-Fluoro-16α,17α-[(r)-(1′-α-furylmethylidene)dioxy]-19-norpregn-4-ene-3,20-dione. J. Nucl. Med. 2012, 53, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Waseda, N.; Kato, Y.; Imura, H.; Kurata, M. Effects of tamoxifen on estrogen and progesterone receptors in human breast cancer. Cancer Res. 1981, 41, 1984–1988. [Google Scholar] [PubMed]
- Fowler, A.M.; Chan, S.R.; Sharp, T.L.; Fettig, N.M.; Zhou, D.; Dence, C.S.; Carlson, K.E.; Jeyakumar, M.; Katzenellenbogen, J.A.; Schreiber, R.D.; et al. Small-animal PET of steroid hormone receptors predicts tumor response to endocrine therapy using a preclinical model of breast cancer. J. Nucl. Med. 2012, 53, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.R.; Fowler, A.M.; Allen, J.A.; Zhou, D.; Dence, C.S.; Sharp, T.L.; Fettig, N.M.; Dehdashti, F.; Katzenellenbogen, J.A. Longitudinal noninvasive imaging of progesterone receptor as a predictive biomarker of tumor responsiveness to estrogen deprivation therapy. Clin. Cancer Res. 2015, 21, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schroder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Perik, P.J.; Lub-De Hooge, M.N.; Gietema, J.A.; van der Graaf, W.T.; de Korte, M.A.; Jonkman, S.; Kosterink, J.G.; van Veldhuisen, D.J.; Sleijfer, D.T.; Jager, P.L.; et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 2006, 24, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, J.E.; Bading, J.R.; Colcher, D.M.; Conti, P.S.; Frankel, P.H.; Carroll, M.I.; Tong, S.; Poku, E.; Miles, J.K.; Shively, J.E.; et al. Functional imaging of human epidermal growth factor receptor 2-positive metastatic breast cancer using 64Cu-DOTA-trastuzumab PET. J. Nucl. Med. 2014, 55, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Gaykema, S.B.; Schroder, C.P.; Vitfell-Rasmussen, J.; Chua, S.; Oude Munnink, T.H.; Brouwers, A.H.; Bongaerts, A.H.; Akimov, M.; Fernandez-Ibarra, C.; Lub-de Hooge, M.N.; et al. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin. Cancer Res. 2014, 20, 3945–3954. [Google Scholar] [CrossRef] [PubMed]
- Gaykema, S.B.; de Jong, J.R.; Perik, P.J.; Brouwers, A.H.; Schroder, C.P.; Oude Munnink, T.H.; Bongaerts, A.H.; de Vries, E.G.; Lub-de Hooge, M.N. 111In-trastuzumab scintigraphy in HER2-positive metastatic breast cancer patients remains feasible during trastuzumab treatment. Mol. Imaging 2014, 13. [Google Scholar] [CrossRef]
- Behr, T.; Behe, M.; Angerstein, C.; Schauer, A.; Kaufmann, C.; Woermann, B.; Becker, W. Does pretherapeutic immunoscintigraphy allow for diagnostic predictions with respect to the toxicity and therapeutic efficacy of cold immunotherapy with trastuzumab (herceptin (R))? J. Nucl. Med. 2000, 41, 73P. [Google Scholar]
- Gebhart, G.; Lamberts, L.E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Prasad, V.; Muller, D.; Schuchardt, C.; Orlova, A.; Wennborg, A.; Tolmachev, V.; Feldwisch, J. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J. Nucl. Med. 2010, 51, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.; Sandberg, D.; Sandstrom, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Astrom, G.; Lubberink, M.; Garske-Roman, U.; Carlsson, J.; et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Vaneycken, I.; Devoogdt, N.; van Gassen, N.; Vincke, C.; Xavier, C.; Wernery, U.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J. 2011, 25, 2433–2446. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.; Blykers, A.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Lahoutte, T.; Devoogdt, N.; Caveliers, V. 18F-nanobody for PET imaging of HER2 overexpressing tumors. Nucl. Med. Biol. 2016, 43, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; McDougald, D.; Choi, J.; Koumarianou, E.; Weitzel, D.; Osada, T.; Lyerly, H.K.; Zalutsky, M.R. Preclinical evaluation of 18F-labeled anti-HER2 nanobody conjugates for imaging HER2 receptor expression by immuno-PET. J. Nucl. Med. 2016, 57, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith-Jones, P.M.; Solit, D.; Afroze, F.; Rosen, N.; Larson, S.M. Early tumor response to HSP90 therapy using HER2 PET: Comparison with 18F-FDG PET. J. Nucl. Med. 2006, 47, 793–796. [Google Scholar] [PubMed]
- Mendler, C.T.; Gehring, T.; Wester, H.J.; Schwaiger, M.; Skerra, A. 89Zr-labeled versus 124I-labeled αHER2 fab with optimized plasma half-life for high-contrast tumor imaging in vivo. J. Nucl. Med. 2015, 56, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Hisada, H.; Temma, T.; Shimizu, Y.; Kimura, H.; Ono, M.; Nakamoto, Y.; Togashi, K.; Saji, H. Gallium-68-labeled anti-HER2 single-chain Fv fragment: Development and in vivo monitoring of HER2 expression. Mol. Imaging Biol. 2015, 17, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oude Munnink, T.H.; de Vries, E.G.; Vedelaar, S.R.; Timmer-Bosscha, H.; Schroder, C.P.; Brouwers, A.H.; Lub-de Hooge, M.N. Lapatinib and 17AAG reduce 89Zr-trastuzumab-F(ab’)2 uptake in SKBR3 tumor xenografts. Mol. Pharm. 2012, 9, 2995–3002. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Scollard, D.; Chen, P.; Wang, J.; Holloway, C.; Reilly, R.M. Imaging of HER2/neu expression in BT-474 human breast cancer xenografts in athymic mice using [99mTc]-HYNIC-trastuzumab (Herceptin) Fab fragments. Nucl. Med. Commun. 2005, 26, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Beylergil, V.; Morris, P.G.; Smith-Jones, P.M.; Modi, S.; Solit, D.; Hudis, C.A.; Lu, Y.; O’Donoghue, J.; Lyashchenko, S.K.; Carrasquillo, J.A.; et al. Pilot study of 68Ga-DOTA-F(ab’)2-trastuzumab in patients with breast cancer. Nucl. Med. Commun. 2013, 34, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Holloway, C.M.; Scollard, D.A.; Caldwell, C.B.; Ehrlich, L.; Kahn, H.J.; Reilly, R.M. Phase I trial of intraoperative detection of tumor margins in patients with HER2-positive carcinoma of the breast following administration of 111In-DTPA-trastuzumab fab fragments. Nucl. Med. Biol. 2013, 40, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Varmira, K.; Hosseinimehr, S.J.; Noaparast, Z.; Abedi, S.M. An improved radiolabelled rna aptamer molecule for HER2 imaging in cancers. J. Drug Target. 2014, 22, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Waser, B.; Foekens, J.A.; Klijn, J.G.; Lamberts, S.W.; Laissue, J. Somatostatin receptor incidence and distribution in breast cancer using receptor autoradiography: Relationship to EGF receptors. Int. J. Cancer 1990, 46, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Orlando, C.; Raggi, C.C.; Bianchi, S.; Distante, V.; Simi, L.; Vezzosi, V.; Gelmini, S.; Pinzani, P.; Smith, M.C.; Buonamano, A.; et al. Measurement of somatostatin receptor subtype 2 mRNA in breast cancer and corresponding normal tissue. Endocr. Relat. Cancer 2004, 11, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Chereau, E.; Durand, L.; Frati, A.; Prignon, A.; Talbot, J.N.; Rouzier, R. Correlation of immunohistopathological expression of somatostatin receptor-2 in breast cancer and tumor detection with 68Ga-DOTATOC and 18F-FDG PET imaging in an animal model. Anticancer Res. 2013, 33, 3015–3019. [Google Scholar] [PubMed]
- Van Eijck, C.H.; Krenning, E.P.; Bootsma, A.; Oei, H.Y.; van Pel, R.; Lindemans, J.; Jeekel, J.; Reubi, J.C.; Lamberts, S.W. Somatostatin-receptor scintigraphy in primary breast cancer. Lancet 1994, 343, 640–643. [Google Scholar] [CrossRef] [Green Version]
- Bajc, M.; Ingvar, C.; Palmer, J. Dynamic indium-111-pentetreotide scintigraphy in breast cancer. J. Nucl. Med. 1996, 37, 622–626. [Google Scholar] [PubMed]
- Chiti, A.; Agresti, R.; Maffioli, L.S.; Tomasic, G.; Savelli, G.; Crippa, F.; Pilotti, S.; Greco, M.; Bombardieri, E. Breast cancer staging using technetium-99m sestamibi and indium-111 pentetreotide single-photon emission tomography. Eur. J. Nucl. Med. 1997, 24, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Vural, G.; Unlu, M.; Atasever, T.; Ozur, I.; Ozdemir, A.; Gokcora, N. Comparison of indium-111 octreotide and thallium-201 scintigraphy in patients mammographically suspected of having breast cancer: Preliminary results. Eur. J. Nucl. Med. 1997, 24, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Alberini, J.L.; Meunier, B.; Denzler, B.; Devillers, A.; Tass, P.; Dazord, L.; Le Simple, T.; Laissue, J.; de Jong, R.; Le Cloirec, J.; et al. Somatostatin receptor in breast cancer and axillary nodes: Study with scintigraphy, histopathology and receptor autoradiography. Breast Cancer Res. Treat. 2000, 61, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Helmholz, T.; Schmitt, J.; Franke, K.; Otto, H.J.; Weise, W. True positive somatostatin receptor scintigraphy in primary breast cancer correlates with expression of sst2A and sst5. Breast Cancer Res. Treat. 2002, 72, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Skanberg, J.; Ahlman, H.; Benjegard, S.A.; Fjalling, M.; Forssell-Aronsson, E.B.; Hashemi, S.H.; Nilsson, O.; Suurkula, M.; Jansson, S. Indium-111-octreotide scintigraphy, intraoperative γ-detector localisation and somatostatin receptor expression in primary human breast cancer. Breast Cancer Res. Treat. 2002, 74, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bossche, B.; Van Belle, S.; de Winter, F.; Signore, A.; van de Wiele, C. Early prediction of endocrine therapy effect in advanced breast cancer patients using 99mTtc-depreotide scintigraphy. J. Nucl. Med. 2006, 47, 6–13. [Google Scholar] [PubMed]
- Wang, F.; Wang, Z.; Wu, J.; Qu, W.; Yao, W.; Zhao, J.; Liu, Z. The role of technetium-99m-labeled octreotide acetate scintigraphy in suspected breast cancer and correlates with expression of sstr. Nucl. Med. Biol. 2008, 35, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Su, X.H.; He, X.J.; Wu, H.; Pan, W.M.; Huang, J.X.; Yu, H.; Chen, G.B.; Wang, W. Complement of tc-99m-octreotide scintimammography to mammography in evaluating breast cancers. Nucl. Sci. Technol. 2010, 21, 24–28. [Google Scholar]
- Piperkova, E. Somatostatin-receptor scintigraphy—A new diagnostic approach to primary breast cancer. Rentgenol. Radiol. 1996, 35, 44–48. [Google Scholar]
- Dalm, S.U.; Melis, M.; Emmering, J.; Kwekkeboom, D.J.; de Jong, M. Breast cancer imaging using radiolabelled somatostatin analogues. Nucl. Med. Biol. 2016, 43, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Dalm, S.U.; Sieuwerts, A.M.; Look, M.P.; Melis, M.; van Deurzen, C.H.; Foekens, J.A.; de Jong, M.; Martens, J.W. Clinical relevance of targeting the gastrin-releasing peptide receptor, somatostatin receptor 2, or chemokine C-X-C motif receptor 4 in breast cancer for imaging and therapy. J. Nucl. Med. 2015, 56, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Grigorakis, S.I.; Watt, H.L.; Sasi, R.; Snell, L.; Watson, P.; Chaudhari, S. Somatostatin receptors in primary human breast cancer: Quantitative analysis of mrna for subtypes 1–5 and correlation with receptor protein expression and tumor pathology. Breast Cancer Res. Treat. 2005, 92, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bossche, B.; D’Haeninck, E.; de Vos, F.; Dierckx, R.A.; van Belle, S.; Bracke, M.; van de Wiele, C. Oestrogen-mediated regulation of somatostatin receptor expression in human breast cancer cell lines assessed with 99mTc-depreotide. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.A.; Alturaihi, H.; Kumar, U. Differential regulation of somatostatin receptors 1 and 2 mRNA and protein expression by tamoxifen and estradiol in breast cancer cells. J. Carcinog. 2005, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalm, S.U.; Schrijver, W.A.M.E.; Sieuwerts, A.M.; Look, M.P.; Ziel-van der Made, A.C.J.; de Weerd, V.; Martens, J.W.; van Diest, P.J.; de Jong, M.; van Deurzen, C.H.M. Prospects of targeting the gastrin releasing peptide receptor, the chemokine C–X–C motif receptor type 4 and the somatostatin receptor subtype 2 in primary breast cancer and metastases (op171). In Proceedings of the 29th Annual Congress of the European Association of Nuclear Medicine (EANM’16), Barcelona, Spain, 15–19 October 2016.
- Limouris, G.S.; Poulantzas, V.; Trompoukis, N.; Karfis, I.; Chondrogiannis, S.; Triantafyllou, N.; Gennimata, V.; Moulopoulou, L.E.; Patsouris, E.; Nikou, G.; et al. Comparison of 111in-[DTPA0]octreotide versus non carrier added 177lu- [DOTA0,Tyr3]-octreotate efficacy in patients with GEP-NET treated intra-arterially for liver metastases. Clin. Nucl. Med. 2016, 41, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Dalm, S.U.; Nonnekens, J.; Doeswijk, G.N.; de Blois, E.; van Gent, D.C.; Konijnenberg, M.W.; de Jong, M. Comparison of the therapeutic response to treatment with a 177lu-labeled somatostatin receptor agonist and antagonist in preclinical models. J. Nucl. Med. 2016, 57, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Fani, M.; Fischer, R.; Del Pozzo, L.; Kaul, F.; Krebs, S.; Fischer, R.; Rivier, J.E.; Reubi, J.C.; Maecke, H.R.; et al. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: A pilot study. J. Nucl. Med. 2014, 55, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Fani, M.; Behe, M.; Brink, I.; Rivier, J.E.; Reubi, J.C.; Maecke, H.R.; Weber, W.A. First clinical evidence that imaging with somatostatin receptor antagonists is feasible. J. Nucl. Med. 2011, 52, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Cescato, R.; Waser, B.; Fani, M.; Reubi, J.C. Evaluation of 177Lu-DOTA-sst2 antagonist versus 177Lu-DOTA-sst2 agonist binding in human cancers in vitro. J. Nucl. Med. 2011, 52, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Ginj, M.; Zhang, H.; Waser, B.; Cescato, R.; Wild, D.; Wang, X.; Erchegyi, J.; Rivier, J.; Macke, H.R.; Reubi, J.C. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc. Natl. Acad. Sci. USA 2006, 103, 16436–16441. [Google Scholar] [CrossRef] [PubMed]
- Dalm, S.U.; Haeck, J.; Doeswijk, G.N.; de Blois, E.; van Deurzen, C.H.M.; de Jong, M. The use of somatostatin receptor antagonists may provide a role for receptor-mediated nuclear imaging and therapy of breast cancer (op305). In Proceedings of the 29th Annual Congress of the European Association of Nuclear Medicine (EANM’16), Barcelona, Spain, 15–19 October 2016.
- Maina, T.; Bergsma, H.; Kulkarni, H.R.; Mueller, D.; Charalambidis, D.; Krenning, E.P.; Nock, B.A.; de Jong, M.; Baum, R.P. Preclinical and first clinical experience with the gastrin-releasing peptide receptor-antagonist [68Ga]SB3 and PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Wenger, S.; Schmuckli-Maurer, J.; Schaer, J.C.; Gugger, M. Bombesin receptor subtypes in human cancers: Detection with the universal radioligand 125I-[d-Tyr(6), β-Ala(11), Phe(13), Nle(14)] bombesin(6–14). Clin. Cancer Res. 2002, 8, 1139–1146. [Google Scholar] [PubMed]
- Reubi, C.; Gugger, M.; Waser, B. Co-expressed peptide receptors in breast cancer as a molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Gugger, M.; Reubi, J.C. Gastrin-releasing peptide receptors in non-neoplastic and neoplastic human breast. Am. J. Pathol. 1999, 155, 2067–2076. [Google Scholar] [CrossRef]
- Dalm, S.U.; Martens, J.W.; Sieuwerts, A.M.; van Deurzen, C.H.; Koelewijn, S.J.; de Blois, E.; Maina, T.; Nock, B.A.; Brunel, L.; Fehrentz, J.A.; et al. In vitro and in vivo application of radiolabeled gastrin-releasing peptide receptor ligands in breast cancer. J. Nucl. Med. 2015, 56, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Mansi, R.; Wang, X.; Forrer, F.; Kneifel, S.; Tamma, M.L.; Waser, B.; Cescato, R.; Reubi, J.C.; Maecke, H.R. Evaluation of a 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-conjugated bombesin-based radioantagonist for the labeling with single-photon emission computed tomography, positron emission tomography, and therapeutic radionuclides. Clin. Cancer Res. 2009, 15, 5240–5249. [Google Scholar] [CrossRef] [PubMed]
- Prignon, A.; Nataf, V.; Provost, C.; Cagnolini, A.; Montravers, F.; Gruaz-Guyon, A.; Lantry, L.E.; Talbot, J.N.; Nunn, A.D. 68Ga-AMBA and 18F-FDG for preclinical PET imaging of breast cancer: Effect of tamoxifen treatment on tracer uptake by tumor. Nucl. Med. Biol. 2015, 42, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Parry, J.J.; Andrews, R.; Rogers, B.E. Micropet imaging of breast cancer using radiolabeled bombesin analogs targeting the gastrin-releasing peptide receptor. Breast Cancer Res. Treat. 2007, 101, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Stoykow, C.; Erbes, T.; Maecke, H.R.; Bulla, S.; Bartholoma, M.; Mayer, S.; Drendel, V.; Bronsert, P.; Werner, M.; Gitsch, G.; et al. Gastrin-releasing peptide receptor imaging in breast cancer using the receptor antagonist 68Ga-RM2 and PET. Theranostics 2016, 6, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Necela, B.M.; Crozier, J.A.; Andorfer, C.A.; Lewis-Tuffin, L.; Kachergus, J.M.; Geiger, X.J.; Kalari, K.R.; Serie, D.J.; Sun, Z.; Moreno-Aspitia, A.; et al. Folate receptor-α (FOLR1) expression and function in triple negative tumors. PLoS ONE 2015, 10, e0122209. [Google Scholar]
- Fisher, R.E.; Siegel, B.A.; Edell, S.L.; Oyesiku, N.M.; Morgenstern, D.E.; Messmann, R.A.; Amato, R.J. Exploratory study of 99mTc-EC20 imaging for identifying patients with folate receptor-positive solid tumors. J. Nucl. Med. 2008, 49, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Nimmagadda, S.; Pullambhatla, M.; Stone, K.; Green, G.; Bhujwalla, Z.M.; Pomper, M.G. Molecular imaging of CXCR4 receptor expression in human cancer xenografts with [64Cu]amd3100 positron emission tomography. Cancer Res. 2010, 70, 3935–3944. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Tian, L.; Cao, X.; Li, L.; Xu, P.; Zhao, C. Imaging CXCR4 expression with 99mTc-radiolabeled small-interference rna in experimental human breast cancer xenografts. Mol. Imaging Biology. 2016, 18, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Vag, T.; Gerngross, C.; Herhaus, P.; Eiber, M.; Philipp-Abbrederis, K.; Graner, F.P.; Ettl, J.; Keller, U.; Wester, H.J.; Schwaiger, M. First experience with chemokine receptor CXCR4-targeted PET imaging of patients with solid cancers. J. Nucl. Med. 2016, 57, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Morgat, C.; Mishra, A.K.; Varshney, R.; Allard, M.; Fernandez, P.; Hindie, E. Targeting neuropeptide receptors for cancer imaging and therapy: Perspectives with bombesin, neurotensin, and neuropeptide-y receptors. J. Nucl. Med. 2014, 55, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Gugger, M.; Waser, B.; Schaer, J.C. Y1-mediated effect of neuropeptide y in cancer: Breast carcinomas as targets. Cancer Res. 2001, 61, 4636–4641. [Google Scholar] [PubMed]
- Khan, I.U.; Zwanziger, D.; Bohme, I.; Javed, M.; Naseer, H.; Hyder, S.W.; Beck-Sickinger, A.G. Breast-cancer diagnosis by neuropeptide y analogues: From synthesis to clinical application. Angew. Chem. 2010, 49, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Maschauer, S.; Kuwert, T.; Beck-Sickinger, A.G.; Prante, O. Synthesis and in vitro and in vivo evaluation of an 18F-labeled neuropeptide y analogue for imaging of breast cancer by PET. Mol. Pharm. 2015, 12, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, Q.; Cheng, L.; Ma, C.; Xiao, L.; Xu, D.; Gao, Y.; Wang, J.; Song, H. NPY1R is a novel peripheral blood marker predictive of metastasis and prognosis in breast cancer patients. Oncol. Lett. 2015, 9, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C. In vitro identification of vasoactive intestinal peptide receptors in human tumors: Implications for tumor imaging. J. Nucl. Med. 1995, 36, 1846–1853. [Google Scholar] [PubMed]
- Zhang, K.; Aruva, M.R.; Shanthly, N.; Cardi, C.A.; Patel, C.A.; Rattan, S.; Cesarone, G.; Wickstrom, E.; Thakur, M.L. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating peptide (PACAP) receptor specific peptide analogues for PET imaging of breast cancer: In vitro/in vivo evaluation. Regul. Pept. 2007, 144, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Jagoda, E.M.; Aloj, L.; Seidel, J.; Lang, L.; Moody, T.W.; Green, S.; Caraco, C.; Daube-Witherspoon, M.; Green, M.V.; Eckelman, W.C. Comparison of an 18F labeled derivative of vasoactive intestinal peptide and 2-deoxy-2-[18F]Fluoro-d-glucose in nude mice bearing breast cancer xenografts. Mol. Imaging Biol. 2002, 4, 369–379. [Google Scholar] [CrossRef]
- Thakur, M.L.; Zhang, K.; Berger, A.; Cavanaugh, B.; Kim, S.; Channappa, C.; Frangos, A.J.; Wickstrom, E.; Intenzo, C.M. VPAC1 receptors for imaging breast cancer: A feasibility study. J. Nucl. Med. 2013, 54, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Virgolini, I.; Kurtaran, A.; Raderer, M.; Leimer, M.; Angelberger, P.; Havlik, E.; Li, S.; Scheithauer, W.; Niederle, B.; Valent, P.; et al. Vasoactive intestinal peptide receptor scintigraphy. J. Nucl. Med. 1995, 36, 1732–1739. [Google Scholar] [CrossRef]
- De Marchi, T.; Foekens, J.A.; Umar, A.; Martens, J.W. Endocrine therapy resistance in estrogen receptor (ER)-positive breast cancer. Drug Discov. Today 2016, 21, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Aurilio, G.; Disalvatore, D.; Pruneri, G.; Bagnardi, V.; Viale, G.; Curigliano, G.; Adamoli, L.; Munzone, E.; Sciandivasci, A.; De Vita, F.; et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur. J. Cancer 2014, 50, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Makki, J. Diversity of breast carcinoma: Histological subtypes and clinical relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Wang, S.H.; Raju, N.; Gierach, I.; Ding, H.; Tweedle, M.F. Heterobivalent dual-target probe for targeting GRP and Y1 receptors on tumor cells. Bioorg. Med. Chem. Lett. 2013, 23, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Lara, L.; Ferro-Flores, G.; Ramirez Fde, M.; Ocampo-Garcia, B.; Santos-Cuevas, C.; Diaz-Nieto, L.; Isaac-Olive, K. Improved radiopharmaceutical based on 99mTc-bombesin-folate for breast tumour imaging. Nucl. Med. Commun. 2016, 37, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Sun, Y.; Chen, B.; Ji, B.; Gao, S.; Ma, Q.; Cheng, G.; Zhang, H. The diagnostic role of 99mTc-dual receptor targeted probe and targeted peptide bombesin (RGD-BBN) SPET/CT in the detection of malignant and benign breast tumors and axillary lymph nodes compared to ultrasound. Hell. J. Nucl. Med. 2015, 18, 108–113. [Google Scholar] [PubMed]
- Liu, Z.; Yan, Y.; Liu, S.; Wang, F.; Chen, X. 18F, 64Cu, and 68Ga labeled RGD-bombesin heterodimeric peptides for PET imaging of breast cancer. Bioconjug. Chem. 2009, 20, 1016–1025. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalm, S.U.; Verzijlbergen, J.F.; De Jong, M. Review: Receptor Targeted Nuclear Imaging of Breast Cancer. Int. J. Mol. Sci. 2017, 18, 260. https://doi.org/10.3390/ijms18020260
Dalm SU, Verzijlbergen JF, De Jong M. Review: Receptor Targeted Nuclear Imaging of Breast Cancer. International Journal of Molecular Sciences. 2017; 18(2):260. https://doi.org/10.3390/ijms18020260
Chicago/Turabian StyleDalm, Simone U., John Fred Verzijlbergen, and Marion De Jong. 2017. "Review: Receptor Targeted Nuclear Imaging of Breast Cancer" International Journal of Molecular Sciences 18, no. 2: 260. https://doi.org/10.3390/ijms18020260
APA StyleDalm, S. U., Verzijlbergen, J. F., & De Jong, M. (2017). Review: Receptor Targeted Nuclear Imaging of Breast Cancer. International Journal of Molecular Sciences, 18(2), 260. https://doi.org/10.3390/ijms18020260